Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse
- PMID: 23792945
- PMCID: PMC3725202
- DOI: 10.1038/nn.3439
Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse
Abstract
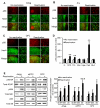
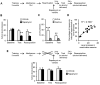
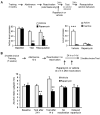
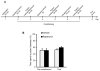
Relapse to alcohol abuse is an important clinical issue that is frequently caused by cue-induced drug craving. Therefore, disruption of the memory for the cue-alcohol association is expected to prevent relapse. It is increasingly accepted that memories become labile and erasable soon after their reactivation through retrieval during a memory reconsolidation process that depends on protein synthesis. Here we show that reconsolidation of alcohol-related memories triggered by the sensory properties of alcohol itself (odor and taste) activates mammalian target of rapamycin complex 1 (mTORC1) in select amygdalar and cortical regions in rats, resulting in increased levels of several synaptic proteins. Furthermore, systemic or central amygdalar inhibition of mTORC1 during reconsolidation disrupts alcohol-associated memories, leading to a long-lasting suppression of relapse. Our findings provide evidence that the mTORC1 pathway and its downstream substrates are crucial in alcohol-related memory reconsolidation and highlight this pathway as a therapeutic target to prevent relapse.
Figures


References
-
- World Health Organization . WHO global status report on alcohol 2004. World Health Organization; Geneva: 2004.
-
- Milton A. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. (in press) - PubMed
-
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. - PubMed
References for Methods section
-
- Puighermanal E, et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

