Amino-terminal domain tetramer organization and structural effects of zinc binding in the N-methyl-D-aspartate (NMDA) receptor
- PMID: 23792960
- PMCID: PMC3829342
- DOI: 10.1074/jbc.M113.482356
Amino-terminal domain tetramer organization and structural effects of zinc binding in the N-methyl-D-aspartate (NMDA) receptor
Abstract
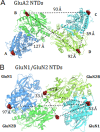
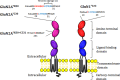
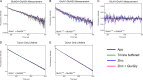
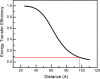
N-Methyl-D-aspartate (NMDA) receptors mediate excitatory neurotransmission in the mammalian central nervous system. An important feature of these receptors is their capacity for allosteric regulation by small molecules, such as zinc, which bind to their amino-terminal domain (ATD). Zinc inhibition through high affinity binding to the ATD has been examined through functional studies; however, there is no direct measurement of associated conformational changes. We used luminescence resonance energy transfer to show that the ATDs undergo a cleft closure-like conformational change upon binding zinc, but no changes are observed in intersubunit distances. Furthermore, we find that the ATDs are more closely packed than the related AMPA receptors. These results suggest that the stability of the upper lobe contacts between ATDs allow for the efficient propagation of the cleft closure conformational change toward the ligand-binding domain and transmembrane segments, ultimately inhibiting the channel.
Keywords: Allosteric Regulation; Amino-terminal Domain; Fluorescence Resonance Energy Transfer (FRET); Glutamate Receptors Ionotropic (AMPA, NMDA); Ion Channels; LRET; Zinc.
Figures



References
-
- Meddows E., Le Bourdelles B., Grimwood S., Wafford K., Sandhu S., Whiting P., McIlhinney R. A. (2001) Identification of molecular determinants that are important in the assembly of N-methyl-d-aspartate receptors. J. Biol. Chem. 276, 18795–18803 - PubMed
-
- Paoletti P., Perin-Dureau F., Fayyazuddin A., Le Goff A., Callebaut I., Neyton J. (2000) Molecular organization of a zinc binding N-terminal modulatory domain in an NMDA receptor subunit. Neuron 28, 911–925 - PubMed
-
- Masuko T., Kashiwagi K., Kuno T., Nguyen N. D., Pahk A. J., Fukuchi J., Igarashi K., Williams K. (1999) A regulatory domain (R1–R2) in the amino terminus of the N-methyl-d-aspartate receptor: effects of spermine, protons, and ifenprodil, and structural similarity to bacterial leucine/isoleucine/valine binding protein. Mol. Pharmacol. 55, 957–969 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

