Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes
- PMID: 23793239
- PMCID: PMC4071061
- DOI: 10.1038/nmeth.2524
Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes
Abstract
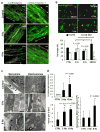
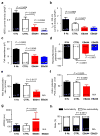
Directed differentiation protocols enable derivation of cardiomyocytes from human pluripotent stem cells (hPSCs) and permit engineering of human myocardium in vitro. However, hPSC-derived cardiomyocytes are reflective of very early human development, limiting their utility in the generation of in vitro models of mature myocardium. Here we describe a platform that combines three-dimensional cell cultivation with electrical stimulation to mature hPSC-derived cardiac tissues. We used quantitative structural, molecular and electrophysiological analyses to explain the responses of immature human myocardium to electrical stimulation and pacing. We demonstrated that the engineered platform allows for the generation of three-dimensional, aligned cardiac tissues (biowires) with frequent striations. Biowires submitted to electrical stimulation had markedly increased myofibril ultrastructural organization, elevated conduction velocity and improved both electrophysiological and Ca(2+) handling properties compared to nonstimulated controls. These changes were in agreement with cardiomyocyte maturation and were dependent on the stimulation rate.
Conflict of interest statement
Figures



References
-
- Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. - PubMed
-
- Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

