Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab
- PMID: 23794225
- PMCID: PMC4238810
- DOI: 10.1002/jbmr.2024
Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab
Abstract
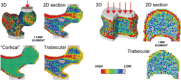
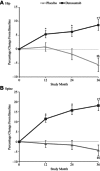
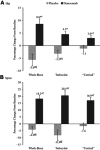
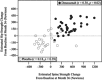
In the randomized, placebo-controlled FREEDOM study of women aged 60 to 90 years with postmenopausal osteoporosis, treatment with denosumab once every 6 months for 36 months significantly reduced hip and new vertebral fracture risk by 40% and 68%, respectively. To gain further insight into this efficacy, we performed a nonlinear finite element analysis (FEA) of hip and spine quantitative computed tomography (QCT) scans to estimate hip and spine strength in a subset of FREEDOM subjects (n = 48 placebo; n = 51 denosumab) at baseline, 12, 24, and 36 months. We found that, compared with baseline, the finite element estimates of hip strength increased from 12 months (5.3%; p < 0.0001) and through 36 months (8.6%; p < 0.0001) in the denosumab group. For the placebo group, hip strength did not change at 12 months and decreased at 36 months (-5.6%; p < 0.0001). Similar changes were observed at the spine: strength increased by 18.2% at 36 months for the denosumab group (p < 0.0001) and decreased by -4.2% for the placebo group (p = 0.002). At 36 months, hip and spine strength increased for the denosumab group compared with the placebo group by 14.3% (p < 0.0001) and 22.4% (p < 0.0001), respectively. Further analysis of the finite element models indicated that strength associated with the trabecular bone was lost at the hip and spine in the placebo group, whereas strength associated with both the trabecular and cortical bone improved in the denosumab group. In conclusion, treatment with denosumab increased hip and spine strength as estimated by FEA of QCT scans compared with both baseline and placebo owing to positive treatment effects in both the trabecular and cortical bone compartments. These findings provide insight into the mechanism by which denosumab reduces fracture risk for postmenopausal women with osteoporosis.
Keywords: DENOSUMAB; FINITE ELEMENT ANALYSIS; HIP STRENGTH; OSTEOPOROSIS; SPINE STRENGTH.
© 2014 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals, Inc. on behalf of the American Society for Bone and Mineral Research.
Figures
References
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. - PubMed
-
- Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478–84. - PubMed
-
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. - PMC - PubMed
-
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

