Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation
- PMID: 23794477
- PMCID: PMC4017598
- DOI: 10.1002/jcb.24609
Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation
Abstract
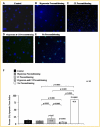
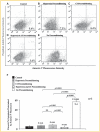
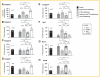
Myocardial infarction is a leading cause of mortality and morbidity worldwide. Occlusion of a coronary artery produces ischemia and myocardial necrosis that leads to left ventricular (LV) remodeling, dysfunction, and heart failure. Stem cell therapy may decrease infarct size and improve LV function; the hypoxic environment, however, following a myocardial infarction may result in apoptosis, which in turn decreases survival of transplanted stem cells. Therefore, the effects of preconditioned mesenchymal stem cells (MSC) with hyperoxia (100% oxygen), Z-VAD-FMK pan-caspase inhibitor (CI), or both in a hypoxic environment in order to mimic conditions seen in cardiac tissue post-myocardial infarction were studied in vitro. MSCs preconditioned with hyperoxia or CI significantly decreased apoptosis as suggested by TUNEL assay and Annexin V analysis using fluorescence assisted cell sorting. These effects were more profound when both, hyperoxia and CI, were used. Additionally, gene and protein expression of caspases 1, 3, 6, 7, and 9 were down-regulated significantly in MSCs preconditioned with hyperoxia, CI, or both, while the survival markers Akt1, NF-κB, and Bcl-2 were significantly increased in preconditioned MSCs. These changes ultimately resulted in a significant increase in MSC proliferation in hypoxic environment as determined by BrdU assays compared to MSCs without preconditioning. These effects may prove to be of great clinical significance when transplanting stem cells into the hypoxic myocardium of post-myocardial infarction patients in order to attenuate LV remodeling and improve LV function.
Keywords: APOPTOSIS; CASPASE INHIBITOR; HYPEROXIA; HYPOXIA; MESENCHYMAL STEM CELLS; PRECONDITIONING.
© 2013 Wiley Periodicals, Inc.
Figures




References
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. - PubMed
-
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. - PubMed
-
- Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, Hagège AA, Menasché P. Transplantation of autologous fresh bone marrow into infarcted myocardium: A word of caution. Circulation. 2003;108(Suppl.I):II-247–II-252. - PubMed
-
- Brazil DP, Hemmings BA. Ten years of protein kinase B signaling: A hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

