A facile glovebox-free strategy to significantly accelerate the syntheses of well-defined polypeptides by N-carboxyanhydride (NCA) ring opening polymerizations
- PMID: 23794753
- PMCID: PMC3686519
- DOI: 10.1021/ma4007939
A facile glovebox-free strategy to significantly accelerate the syntheses of well-defined polypeptides by N-carboxyanhydride (NCA) ring opening polymerizations
Abstract
A facile N2 flow-accelerated N-carboxyanhydride ring opening polymerization (NCA ROP) is demonstrated, herein, with rigorous kinetic studies to evaluate the methodology in detail. By using n-hexylamine as initiator and γ-benzyl-L-glutamate N-carboxyanhydride (BLG-NCA) as monomer, the NCA ROP via a normal amine mechanism (NAM) reached 90% conversion in 2 h under N2 flow at room temperature in a fume hood, much shorter than the time required for the same polymerization conducted in a glove box (14 h). The efficient removal of CO2 from the reaction by N2 flow drove the carbamic acid-amine equilibrium toward the formation of active nucleophilic amino termini and promoted polymerization. The detailed kinetic studies of the polymerization with different feed ratios and N2 flow rates were conducted, demonstrating the living feature of the NCA ROP and the tuning of the polymerization rate by simply changing the flow rate of N2. Maintenance of the reactivity of the amino ω-chain terminus and control during a subsequent polymerization were confirmed by performing chain extension reactions. The N2 flow method provides a new straightforward strategy to synthesize well-defined polypeptides with predictable molecular weights and narrow molecular weight distributions (PDI < 1.19).
Keywords: NCA; Nitrogen flow; ring opening polymerization.
Figures


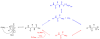
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
