2-Amino-5-bromo-pyridin-1-ium (2-amino-5-bromo-pyridine-κN (1))trichloridozincate
- PMID: 23794973
- PMCID: PMC3684871
- DOI: 10.1107/S1600536813011884
2-Amino-5-bromo-pyridin-1-ium (2-amino-5-bromo-pyridine-κN (1))trichloridozincate
Abstract
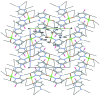
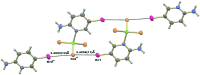
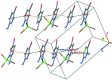
The structure of the title salt, (C5H6BrN2)[ZnCl3(C5H5BrN2)], consists of discrete 2-amino-5-bromo-pyridin-1-ium cations and distorted tetra-hedral (2-amino-5-bromo-pyridine)-tri-chlorido-zincate anions. In the crystal, the complex anions and cations are linked via N-H⋯Cl hydrogen bonds into layers parallel to (101). Short Br⋯Cl contacts of 3.4239 (11) and 3.4503 (12) Å are observed, as well as π-π stacking inter-actions between the pyridine and pyridinium rings, with alternating centroid-to-centroid distances of 3.653 (2) and 3.845 (2) Å.
Figures

References
-
- Bruker (1998). SMART Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2003). SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Hubrich, M., Peukert, M., Seichter, W. & Weber, E. (2010). Polyhedron, 29, 1854–1862.
-
- Janiak, C., Deblon, S., Wu, H., Kolm, M. J., Klüfers, P., Piotrowski, H. & Mayer, P. (1999). Eur. J. Inorg. Chem. pp. 1507–1521.
-
- Jo, Y. W., Im, W. B., Rhee, J. K., Shim, M. J., Kim, W. B. & Choi, E. C. (2004). Bioorg. Med. Chem. pp. 5909–5915. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
