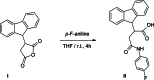
2-(9H-Fluoren-9-yl)-4-(4-fluoro-anilino)-4-oxo-butanoic acid
- PMID: 23795121
- PMCID: PMC3685102
- DOI: 10.1107/S1600536813013779
2-(9H-Fluoren-9-yl)-4-(4-fluoro-anilino)-4-oxo-butanoic acid
Abstract
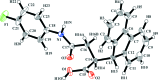
In the title compound, C23H18FNO3, the tricyclic 9-fluorenyl system is approximately planar (r.m.s. deviation = 0.0279 Å). The N-C(=O) bond length is comparatively short [1.359 (3) Å], which is typical for such conjugated systems. The N atom has a planar configuration [sum of bond angles= 359.8°] due to conjugation of its lone pair with the π-system of the carbonyl group. In the crystal, a three-dimensional network is formed through N-H⋯O and O-H⋯O hydrogen bonds between the amide and carb-oxy-lic acid groups and carbonyl O-atom acceptors.
Figures
References
-
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2008). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Clar, E. (1942). Reichsamt Wirtschaftsausbau Chem. Ber., Pruf-Nr. 015(PB52017), pp. 859–878.
-
- Galustyan, G. G., Levkovich, M. G. & Abdullaev, N. D. (2000). Chem. Heterocycl. Compd, 36, 1402–1408.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous