Determination of artemether and dihydroartemisinin in human plasma with a new hydrogen peroxide stabilization method
- PMID: 23795928
- PMCID: PMC3879143
- DOI: 10.4155/bio.13.91
Determination of artemether and dihydroartemisinin in human plasma with a new hydrogen peroxide stabilization method
Abstract
Background: Numerous methods have been reported for the determination of artemether (ARM) and its metabolite dihydroartemisinin (DHA) in plasma. However, stability issues in patient plasma have not received enough attention.
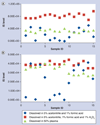
Results: An LC-MS/MS method for simultaneous determination of ARM and DHA in human plasma (K3EDTA) turned out to be problematic: ARM and DHA were degraded partially or completely in some patient plasma samples as indicated by the stable isotope-labeled internal standards. We postulated iron II (Fe(2+)) in hemoglobin or its derived products from malaria patients causes degradation of the drugs, and found that hydrogen peroxide (H2O2) protected the drugs from degradation. Acidifying plasma increased recovery of ARM significantly. Using only 50 µl of plasma sample, the method has a LLOQ at 0.5 ng/ml for both ARM and DHA.
Conclusion: H2O2 is a stabilizing agent for artemisinin derivatives. The modified method is reliable and sensitive.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures


References
-
- Peys E, Vandenkerckhove J, Van Hemel J, Sas B. Simultaneous determination of β-artemether and its metabolite dihydroartemisinin in human plasma and urine by a high-performance liquid chromatography-mass spectrometry assay using electrospray ionisation. Chromatographia. 2005;61:637–641.
-
- Shi B, YU Y, Li Z, et al. Quantitative analysis of artemether and its metabolite dihydroartemisinin in human plasma by LC with tandem mass spectrometry. Chromatographia. 2006;64:523–530.
-
- Souppart C, Gauducheau N, Sandrenan N, Richard F. Development and validation of a high-performance liquid chromatography–mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;774:195–203. - PubMed
-
- Hanpithakpong W, Kamanikom B, Singhasivanon P, White NJ, Day NPJ, Lindegardh N. A liquid chromatographic–tandem mass spectrometric method for determination of artemether and its metabolite dihydroartemisinin in human plasma. Bioanalysis. 2009;1:37–46. - PubMed
-
- Huang L, Jayewardene AL, Li X, Marzan F, Lizak PS, Aweeka FT. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for determination of artemether and its active metabolite dihydroartemisinin in human plasma. J. Pharm. Biomed. Anal. 2009;50:959–965. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
