Amphidynamic crystals of a steroidal bicyclo[2.2.2]octane rotor: a high symmetry group that rotates faster than smaller methyl and methoxy groups
- PMID: 23796326
- PMCID: PMC3963821
- DOI: 10.1021/ja4024463
Amphidynamic crystals of a steroidal bicyclo[2.2.2]octane rotor: a high symmetry group that rotates faster than smaller methyl and methoxy groups
Abstract
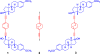
The synthesis, crystallization, single crystal X-ray structure, and solid state dynamics of molecular rotor 3 provided with a high symmetry order and relatively cylindrical bicyclo[2.2.2]octane (BCO) rotator linked to mestranol fragments were investigated in this work. By use of solid state (13)C NMR, three rotating fragments were identified within the molecule: the BCO, the C19 methoxy and the C18 methyl groups. To determine the dynamics of the BCO group in crystals of 3 by variable temperature (1)H spin-lattice relaxation (VT (1)H T1), we determined the (1)H T1 contributions from the methoxy group C19 by carrying out measurements with the methoxy-deuterated isotopologue rotor 3-d6. The contributions from the quaternary methyl group C18 were estimated by considering the differences between the VT (1)H T1 of mestranol 8 and methoxy-deuterated mestranol 8-d3. From these studies it was determined that the BCO rotator in 3 has an activation energy of only 1.15 kcal mol(-1), with a barrier for site exchange that is smaller than those of methyl (E(a) = 1.35 kcal mol(-1)) and methoxy groups (E(a) = 1.92 kcal mol(-1)), despite their smaller moments of inertia and surface areas.
Figures





References
-
- Mislow K. Chemtracts: Org Chem. 1988;2:151.
- Balzani V, Venturi M, Credi A. Molecular Devices and Machines. Wiley-VCH Verlag; Weinheim, Germany: 2003.
- Kelley TR, editor. Molecular Machines in Topics in Current Chemistry. Springer; New York: 2005.
- Coskun A, Banaszak M, Astumian RD, Stoddart JF, Grzybowki BA. Chem Soc Rev. 2012;41:19. - PubMed
-
- Newnham RE. Properties of Materials, Anisotropy, Symmetry and Structure. Oxford University Press; Oxford; 2005.
-
- Desiraju G, Vittal JJ, Ramanan A. Crystal Engineering A Textbook. Word Scientific Publishing Co; Singapore: 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

