Molecular mechanisms of muscle atrophy in myotonic dystrophies
- PMID: 23796888
- PMCID: PMC3759660
- DOI: 10.1016/j.biocel.2013.06.010
Molecular mechanisms of muscle atrophy in myotonic dystrophies
Abstract
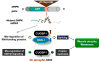
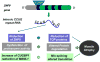
Myotonic dystrophy type 1 (DM1) and myotonic dystrophy type 2 (DM2) are multisystemic diseases that primarily affect skeletal muscle, causing myotonia, muscle atrophy, and muscle weakness. DM1 and DM2 pathologies are caused by expansion of CTG and CCTG repeats in non-coding regions of the genes encoding myotonic dystrophy protein kinase (DMPK) and zinc finger protein 9 (ZNF9) respectively. These expansions cause DM pathologies through accumulation of mutant RNAs that alter RNA metabolism in patients' tissues by targeting RNA-binding proteins such as CUG-binding protein 1 (CUGBP1) and Muscle blind-like protein 1 (MBNL1). Despite overwhelming evidence showing the critical role of RNA-binding proteins in DM1 and DM2 pathologies, the downstream pathways by which these RNA-binding proteins cause muscle wasting and muscle weakness are not well understood. This review discusses the molecular pathways by which DM1 and DM2 mutations might cause muscle atrophy and describes progress toward the development of therapeutic interventions for muscle wasting and weakness in DM1 and DM2. This article is part of a Directed Issue entitled: Molecular basis of muscle wasting.
Keywords: CUG/CCUG repeats; Muscle atrophy; Myotonic dystrophy type 1; Myotonic dystrophy type 2; Therapeutic approaches.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Amack JD, Mahadevan MS. Myogenic defects in myotonic dystrophy. Dev Biol. 2004;265:294–301. - PubMed
-
- Botta A, Caldarola S, Vallo L, Bonifazi E, Fruci D, Gullotta F, et al. Effect of the [CCTG]n repeat expansion on ZNF9 expression in myotonic dystrophy II (DM2) Biochem Biophys Acta. 2006;1762:329–34. - PubMed
-
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeats at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

