Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection
- PMID: 23796953
- PMCID: PMC4031445
- DOI: 10.1016/j.tips.2013.05.003
Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection
Abstract
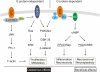
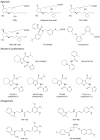
Modulation of a specific prostanoid synthase or receptor provides therapeutic alternatives to nonsteroidal anti-inflammatory drugs (NSAIDs) for treating pathological conditions governed by cyclooxygenase-2 (COX-2 or PTGS2). Among the COX-2 downstream signaling pathways, the prostaglandin E2 (PGE2) receptor EP2 subtype (PTGER2) is emerging as a crucial mediator of many physiological and pathological events. Genetic ablation strategies and recent advances in chemical biology provide tools for a better understanding of EP2 signaling. In the brain, the EP2 receptor modulates some beneficial effects, including neuroprotection, in acute models of excitotoxicity, neuroplasticity, and spatial learning via cAMP-PKA signaling. Conversely, EP2 activation accentuates chronic inflammation mainly through the cAMP-Epac pathway, likely contributing to delayed neurotoxicity. EP2 receptor activation also engages β-arrestin in a G-protein-independent pathway that promotes tumor cell growth and migration. Understanding the conditions under which multiple EP2 signaling pathways are engaged might suggest novel therapeutic strategies to target this key inflammatory prostaglandin receptor.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Grosser T, et al. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annual review of medicine. 2010;61:17–33. - PubMed
-
- Samuelsson B, et al. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. - PubMed
-
- Barco A, Kandel ER. The Role of CREB and CBP in Brain Function. In: Thiel G, editor. Transcription Factors in the Nervous System. Wiley-VCH Verlag GmbH & Co.; 2006. pp. 207–241.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

