BAFF- and APRIL-dependent maintenance of antibody titers after immunization with T-dependent antigen and CD1d-binding ligand
- PMID: 23797666
- PMCID: PMC3720783
- DOI: 10.4049/jimmunol.1300263
BAFF- and APRIL-dependent maintenance of antibody titers after immunization with T-dependent antigen and CD1d-binding ligand
Abstract
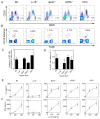
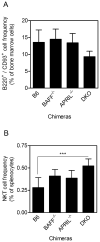
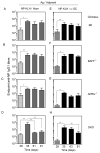
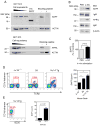
CD1d-restricted invariant NKT (iNKT) cells boost humoral immunity to T-dependent Ags that are coadministered with the CD1d-binding glycolipid Ag α-galactosylceramide (α-GC). Observations that mice lacking iNKT cells have decaying Ab responses following vaccination have led to the hypothesis that iNKT cells express plasma cell (PC) survival factors that sustain specific Ab titers. Bone marrow chimeric mice in which the entire hematopoietic compartment or iNKT cells selectively lacked BAFF, a proliferation-inducing ligand (APRIL), or both BAFF and APRIL were created and immunized with nitrophenol hapten-conjugated keyhole limpet hemocyanin adsorbed to Imject aluminum hydroxide-containing adjuvant or mixed with α-GC. In comparison with BAFF- or APRIL-sufficient bone marrow chimeras, absence of hematopoietic compartment- and iNKT-derived BAFF and APRIL was associated with rapidly decaying Ab titers and reduced PC numbers. The iNKT cell-derived BAFF or APRIL assumed a greater role in PC survival when α-GC was used as the adjuvant for immunization. These results show that iNKT cell-derived BAFF and APRIL each contribute to survival of PCs induced by immunization. This study sheds new light on the mechanisms through which iNKT cells impact humoral immunity and may inform design of vaccines that incorporate glycolipid adjuvants.
Figures




References
-
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annual review of immunology. 2003;21:483–513. - PubMed
-
- Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. The Journal of experimental medicine. 2011;208:1163–1177. - PMC - PubMed
-
- Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nature immunology. 2002;3:867–874. - PubMed
-
- Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. The Journal of experimental medicine. 2003;198:267–279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

