Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults
- PMID: 23797677
- PMCID: PMC10373438
- DOI: 10.1002/14651858.CD010611
Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults
Abstract
Background: Some antiepileptic drugs but not others are useful in clinical practice for the prophylaxis of migraine. This might be explained by the variety of actions of these drugs in the central nervous system. The present review is part of an update of a Cochrane review first published in 2004, and previously updated (conclusions not changed) in 2007.
Objectives: To describe and assess the evidence from controlled trials on the efficacy and tolerability of valproate (valproic acid or sodium valproate or a combination of the two) for preventing migraine attacks in adult patients with episodic migraine.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2012, Issue 12), PubMed/MEDLINE (1966 to 15 January 2013), MEDLINE In-Process (current week, 15 January 2013), and EMBASE (1974 to 15 January 2013) and handsearched Headache and Cephalalgia through January 2013.
Selection criteria: Studies were required to be prospective, controlled trials of valproate taken regularly to prevent the occurrence of migraine attacks, to improve migraine-related quality of life, or both.
Data collection and analysis: Two review authors independently selected studies and extracted data. For headache frequency data, we calculated mean differences (MDs) between valproate and comparator (placebo, active control, or valproate in a different dose) for individual studies and pooled these across studies. For dichotomous data on responders (patients with ≥ 50% reduction in headache frequency), we calculated odds ratios (ORs) and, in select cases, risk ratios (RRs); we also calculated numbers needed to treat (NNTs). We calculated MDs for Migraine Disability Assessment (MIDAS) scores. We also summarised data on adverse events from placebo-controlled trials and calculated risk differences (RDs) and numbers needed to harm (NNHs).
Main results: Ten papers describing 10 unique trials met the inclusion criteria. Analysis of data from two trials (63 participants) showed that sodium valproate reduced headache frequency by approximately four headaches per 28 days as compared to placebo (MD -4.31; 95% confidence interval (CI) -8.32 to -0.30). Data from four trials (542 participants) showed that divalproex sodium (a stable combination of sodium valproate and valproic acid in a 1:1 molar ratio) more than doubled the proportion of responders relative to placebo (RR 2.18; 95% CI 1.28 to 3.72; NNT 4; 95% CI 2 to 11). One study of sodium valproate (34 participants) versus placebo supported the latter findings (RR for responders 2.83; 95% CI 1.27 to 6.31; NNT 3; 95% CI 2 to 9). There was no significant difference in the proportion of responders between sodium valproate versus flunarizine (one trial, 41 participants) or between divalproex sodium versus propranolol (one trial, 32 participants). Pooled analysis of post-treatment mean headache frequencies in two trials (88 participants) demonstrates a slight but significant advantage for topiramate 50 mg over valproate 400 mg (MD -0.90; 95% CI -1.58 to -0.22). For placebo-controlled trials of sodium valproate and divalproex sodium, NNHs for clinically important adverse events ranged from 7 to 14.
Authors' conclusions: Valproate is effective in reducing headache frequency and is reasonably well tolerated in adult patients with episodic migraine.
Conflict of interest statement
Prof Linde: During the process of preparing this review the author received a travel grant from Allergan in Sweden and was involved as an investigator in a clinical trial in Norway sponsored by AstraZeneca and comparing candesartan, propranolol, and placebo in the prophylaxis of migraine.
Dr Mulleners: The author was a paid consultant for the Merck Dutch Migraine Advisory Board and received a speaker's fee from Merck Sharp & Dohme Corp.
Prof Chronicle: Author deceased. During the process of preparing the original review the author was a paid consultant for Johnson & Johnson and NPS Pharmaceuticals in the USA.
Assoc Prof McCrory: During 2008, the author was a paid expert witness for the plaintiffs in a legal action against the manufacturer of Neurontin (gabapentin). In this capacity, he prepared a systematic review examining previously confidential research reports obtained from the manufacturer (through discovery), along with published trial reports of gabapentin for migraine prophylaxis, and testified at trial.
Figures
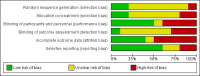
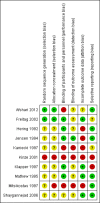
















References
References to studies included in this review
Afshari 2012 {published data only (unpublished sought but not used)}
-
- Afshari D, Rafizadeh S, Rezaei M. A comparative study of the effects of low‐dose topiramate versus sodium valproate in migraine prophylaxis. International Journal of Neuroscience 2012;122(2):60‐8. [MEDLINE: ] - PubMed
Freitag 2002 {published data only}
-
- Freitag FG, Collins SD, Carlson HA, Goldstein J, Saper J, Silberstein S, et al. A randomized trial of divalproex sodium extended‐release tablets in migraine prophylaxis. Neurology 2002;58(11):1652‐9. [MEDLINE: ] - PubMed
Hering 1992 {published data only}
-
- Hering R, Kuritzky A. Sodium valproate in the prophylactic treatment of migraine: a double‐blind study versus placebo. Cephalalgia 1992;12(2):81‐4. [MEDLINE: ] - PubMed
Jensen 1994 {published data only}
-
- Jensen R, Brinck T, Olesen J. Sodium valproate has a prophylactic effect in migraine without aura: a triple‐blind, placebo‐controlled crossover study. Neurology 1994;44(4):647‐51. [MEDLINE: ] - PubMed
Kaniecki 1997 {published data only}
-
- Kaniecki RG. A comparison of divalproex with propanolol and placebo for the prophylaxis of migraine without aura. Archives of Neurology 1997;54(9):1141‐5. [MEDLINE: ] - PubMed
Kinze 2001 {published data only}
-
- Kinze S, Clauss M, Reuter U, Wolf T, Dreier JP, Einhaupl KM, et al. Valproic acid is effective in migraine prophylaxis at low serum levels: a prospective open‐label study. Headache 2001;41(8):774‐8. [MEDLINE: ] - PubMed
Klapper 1997 {published data only}
-
- Klapper J, on behalf of the Divalproex Sodium in Migraine Prophylaxis Study Group. Divalproex sodium in migraine prophylaxis: a dose‐controlled study. Cephalalgia 1997;17(2):103‐8. [MEDLINE: ] - PubMed
Mathew 1995 {published data only}
-
- Mathew NT, Saper JR, Silberstein SD, Rankin L, Markley HG, Solomon S, et al. Migraine prophylaxis with divalproex. Archives of Neurology 1995;52(3):281‐6. [MEDLINE: ] - PubMed
Mitsikostas 1997 {published data only}
-
- Mitsikostas DD, Polychronidis I. Valproate versus flunarizine in migraine prophylaxis: a randomized, double‐open, clinical trial. Functional Neurology 1997;12(5):267‐76. [MEDLINE: ] - PubMed
Shaygannejad 2006 {published data only}
-
- Shaygannejad V, Janghorbani M, Ghorbani A, Ashtary F, Zakizade N, Nasr V. Comparison of the effect of topiramate and sodium valproate in migraine prevention: a randomized blinded crossover study. Headache 2006;46(4):642‐8. [MEDLINE: ] - PubMed
References to studies excluded from this review
Arnold 1998 {published data only}
-
- Arnold G, Einhaupl KM. Valproic acid in the prophylactic treatment of migraine [Valproinsaure in der prophylaktischen Behandlung der Migräne]. Nervenarzt 1998;69(10):913‐8. [MEDLINE: ] - PubMed
Bartolini 2005 {published data only}
-
- Bartolini M, Silvestrini M, Taffi R, Lanciotti C, Luconi R, Capecci M, et al. Efficacy of topiramate and valproate in chronic migraine. Clinical Neuropharmacology 2005;28(6):277‐9. [MEDLINE: ] - PubMed
Bavrasad 2010 {published data only}
-
- Bavrasad R, Nejad SEM, Yarahmadi AR, Sajedi SI, Rahim F. Assessment of the middle dose of topiramate in comparison with sodium valproate for migraine prophylaxis: a randomized double‐blind study. International Journal of Pharmacology 2010;6(5):670‐5. [EMBASE: 2010449325]
Blumenfeld 2008 {published data only}
-
- Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache 2008;48(2):210‐20. [MEDLINE: ] - PubMed
Cutrer 2001 {published data only}
-
- Cutrer FM. Antiepileptic drugs: how they work in headache. Headache 2001;41 Suppl 1:S3‐S10. - PubMed
Erdemoglu 2000 {published data only}
-
- Erdemoglu AK, Ozbakir S. Valproic acid in prophylaxis of refractory migraine. Acta Neurologica Scandinavica 2000;102(6):354‐8. [MEDLINE: ] - PubMed
Fragoso 2003 {published data only}
-
- Fragoso YD. Low dose of sodium divalproate for the treatment of migraine. MedGenMed [electronic resource]: Medscape General Medicine 2003;5(1):32. [MEDLINE: ] - PubMed
Ghose 1998 {published data only}
-
- Ghose K, Niven B. Prophylactic sodium valproate therapy in patients with drug‐resistant migraine. Methods & Findings in Experimental & Clinical Pharmacology 1998;20(4):353‐9. [MEDLINE: ] - PubMed
Green 2005 {published data only}
-
- Green MW, Giordano S, Jiang P, Jafari M, Smith TB. Effect of divalproex on metabolic parameters is dose related in migraine prophylaxis. Headache 2005;45(8):1031‐7. [MEDLINE: ] - PubMed
Keyvan 2009 {published and unpublished data}
-
- Keyvan G, Abolfazl MB. Comparison of treatment effect of sodium valproate, propranolol and tricyclic antidepressants in migraine. Pakistan Journal of Biological Sciences 2009;12(15):1098‐101. [MEDLINE: ] - PubMed
Klapper 1994 {published data only}
-
- Klapper JA. An open label cross‐over comparison of divalproex sodium and propanolol HCl in the prevention of migraine headaches. Headache Quarterly 1994;5(4):50‐3.
Krymchantowski 2011 {published data only}
-
- Krymchantowski AV, Jevoux CC. Topiramate vs divalproex sodium in the preventive treatment of migraine: a prospective "real‐world" study. Headache 2011;51(4):554‐8. [EMBASE: 2011188025] - PubMed
Lenaerts 1996 {published data only}
-
- Lenaerts M, Bastings E, Sianard J, Schoenen J. Sodium valproate in severe migraine and tension‐type headache: an open study of long‐term efficacy and correlation with blood levels. Acta Neurologica Belgica 1996;96(2):126‐9. [MEDLINE: ] - PubMed
Millan‐Guerrero 2007 {published data only}
-
- Millan‐Guerrero RO, Isais‐Millan R, Barreto‐Vizcaino S, Rivera‐Castano L, Garcia‐Solorzano A, Lopez‐Blanca C, et al. Subcutaneous histamine versus sodium valproate in migraine prophylaxis: a randomized, controlled, double‐blind study. European Journal of Neurology 2007;14(10):1079‐84. [MEDLINE: ] - PubMed
Rothrock 1994 {published data only}
-
- Rothrock JF, Kelly NM, Brody ML, Golbeck A. A differential response to treatment with divalproex sodium in patients with intractable headache. Cephalalgia 1994;14(3):241‐4. [MEDLINE: ] - PubMed
Rothrock 1997 {published data only}
-
- Rothrock JF. Clinical studies of valproate for migraine prophylaxis. Cephalalgia 1997;17(2):81‐3. [MEDLINE: ] - PubMed
Silberstein 1993 {published data only}
-
- Silberstein SD, Saper JR, Mathew NT. The safety and efficacy of divalproex sodium in the prophylaxis of migraine headache: a multicenter, double‐blind, placebo‐controlled trial. Headache 1993;33(5):264‐5.
Sørensen 1988 {published data only}
-
- Sørensen KV. Valproate: a new drug in migraine prophylaxis. Acta Neurologica Scandinavica 1988;78(4):346‐8. [MEDLINE: ] - PubMed
Additional references
Ad Hoc Cttee 1962
-
- Ad Hoc Committee on the Classification of Headache of the National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA 1962;179(9):717‐8.
Alderson 2004
-
- Alderson P, Green S, Higgins JPT, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]; Appendix 5b. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Clarke 1996
-
- Clarke CE, MacMillan L, Sondhi S, Wells NE. Economic and social impact of migraine. QJM 1996;89(1):77‐84. [MEDLINE: ] - PubMed
Cutrer 1997
-
- Cutrer FM, Limmroth V, Moskowitz MA. Possible mechanisms of valproate in migraine prophylaxis. Cephalalgia 1997;17(2):93‐100. - PubMed
Dalkara 2012
-
- Dalkara S, Karakurt A. Recent progress in anticonvulsant drug research: strategies for anticonvulsant drug development and applications of antiepileptic drugs for non‐epileptic central nervous system disorders. Current Topics in Medicinal Chemistry 2012;12(9):1033‐71. [MEDLINE: ] - PubMed
Edmeads 1993
-
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. [MEDLINE: ] - PubMed
Edvinsson 2010
-
- Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet 2010;376(9741):645‐55. [MEDLINE: ] - PubMed
Evers 2009
-
- Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. European Journal of Neurology 2009;16(9):968‐81. [MEDLINE: ] - PubMed
GBD 2010 Study
ICHD‐II 2004
-
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. - PubMed
IHS Cttee 1988
-
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [MEDLINE: ] - PubMed
Linde 2004
Linde 2012
-
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [MEDLINE: ] - PubMed
Linde 2013a
Linde 2013b
Linde 2013c
Linde 2013d
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998.
Mehuys 2012
-
- Mehuys E, Paemeleire K, Hees T, Christiaens T, Bortel LM, Tongelen I, et al. Self‐medication of regular headache: a community pharmacy‐based survey. European Journal of Neurology 2012;19(8):1093‐9. [MEDLINE: ] - PubMed
Morrell 2003
-
- Morrell MJ. Reproductive and metabolic disorders in women with epilepsy. Epilepsia 2003;44 Suppl 4:11‐20. - PubMed
Olesen 2006
-
- Olesen J, Bousser M‐G, Diener H‐C, Dodick D, First M, Goadsby PJ, et al. Headache Classification Committee. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006;26(6):742‐6. [MEDLINE: ] - PubMed
Porter 1992
-
- Porter RJ, Meldrum BS. Antiepileptic drugs. In: Katzung BG editor(s). Basic and Clinical Pharmacology. 5th Edition. Norwalk, CT: Appleton & Lange, 1992:331‐49.
Reveiz‐Herault 2003
-
- Reveiz‐Herault L, Cardona AF, Ospina EG, Carrillo P. Effectiveness of flunarizine in the prophylaxis of migraine: a meta‐analytical review of the literature [Eficacia de flunaricina en la profilaxis de migrana: revision metaanalitica de la bibliografia]. Revista de Neurologia 2003;36(10):907‐12. [MEDLINE: ] - PubMed
Silberstein 2008
-
- Silberstein S, Saper J, Berenson F, Somogyi M, McCague K, D'Souza J. Oxcarbazepine in migraine headache: a double‐blind, randomized, placebo‐controlled study. Neurology 2008;70(7):548‐55. [MEDLINE: ] - PubMed
Silberstein 2012
-
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E, Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence‐based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78(17):1337‐45. [MEDLINE: ] - PMC - PubMed
Tfelt‐Hansen 2012
-
- Tfelt‐Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, et al. International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32(1):6‐38. [MEDLINE: ] - PubMed
Victor 2003
References to other published versions of this review
Chronicle 2004
Mulleners 2008
-
- Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia 2008;28(6):585‐97. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

