Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries
- PMID: 23797856
- PMCID: PMC3760626
- DOI: 10.1038/cr.2013.83
Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries
Abstract
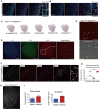
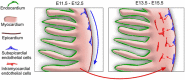
Coronary arteries bring blood flow to the heart muscle. Understanding the developmental program of the coronary arteries provides insights into the treatment of coronary artery diseases. Multiple sources have been described as contributing to coronary arteries including the proepicardium, sinus venosus (SV), and endocardium. However, the developmental origins of coronary vessels are still under intense study. We have produced a new genetic tool for studying coronary development, an AplnCreER mouse line, which expresses an inducible Cre recombinase specifically in developing coronary vessels. Quantitative analysis of coronary development and timed induction of AplnCreER fate tracing showed that the progenies of subepicardial endothelial cells (ECs) both invade the compact myocardium to form coronary arteries and remain on the surface to produce veins. We found that these subepicardial ECs are the major sources of intramyocardial coronary vessels in the developing heart. In vitro explant assays indicate that the majority of these subepicardial ECs arise from endocardium of the SV and atrium, but not from ventricular endocardium. Clonal analysis of Apln-positive cells indicates that a single subepicardial EC contributes equally to both coronary arteries and veins. Collectively, these data suggested that subepicardial ECs are the major source of intramyocardial coronary arteries in the ventricle wall, and that coronary arteries and veins have a common origin in the developing heart.
Figures
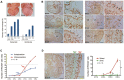


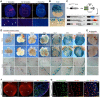
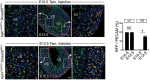
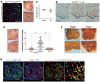

Comment in
-
The mysterious origins of coronary vessels.Cell Res. 2013 Sep;23(9):1063-4. doi: 10.1038/cr.2013.90. Epub 2013 Jul 9. Cell Res. 2013. PMID: 23835479 Free PMC article.
References
-
- Riley P, Smart N. Vascularizing the heart. Cardiovasc Res. 2011;91:260–268. - PubMed
-
- Riley P. Developmental biology: Plumbing the heart. Nature. 2010;464:498–499. - PubMed
-
- Hutchins G, Kessler-Hanna A, Moore G. Development of the coronary arteries in the embryonic human heart. Circulation. 1988;77:1250–1257. - PubMed
-
- Bogers A, Gittenberger-de Groot A, Poelmann R, Peault B, Huysmans H. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth. Anat Embryol (Berl) 1989;180:437–441. - PubMed
-
- Majesky M. Development of coronary vessels. Curr Top Dev Biol. 2004;62:225–259. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

