Oncogene-induced cellular senescence elicits an anti-Warburg effect
- PMID: 23798001
- PMCID: PMC3867201
- DOI: 10.1002/pmic.201200298
Oncogene-induced cellular senescence elicits an anti-Warburg effect
Abstract
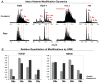
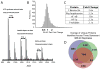
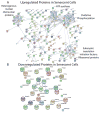
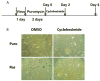
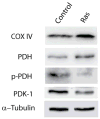
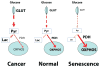
Cellular senescence, an irreversible cell cycle arrest induced by a diversity of stimuli, has been considered as an innate tumor suppressing mechanism with implications and applications in cancer therapy. Using a targeted proteomics approach, we show that fibroblasts induced into senescence by expression of oncogenic Ras exhibit a decrease of global acetylation on all core histones, consistent with formation of senescence-associated heterochromatic foci. We also detected clear increases in repressive markers (e.g. >50% elevation of H3K27me2/3) along with decreases in histone marks associated with increased transcriptional expression/elongation (e.g. H3K36me2/3). Despite the increases in repressive marks of chromatin, 179 loci (of 2206 total) were found to be upregulated by global quantitative proteomics. The changes in the cytosolic proteome indicated an upregulation of mitochondrial proteins and downregulation of proteins involved in glycolysis. These alterations in primary metabolism are opposite to the well-known Warburg effect observed in cancer cells. This study significantly improves our understanding of stress-induced senescence and provides a potential application for triggering it in antiproliferative strategies that target the primary metabolism in cancer cells.
Keywords: Cell biology; Histones; Metabolism; Oxidative phosphorylation; Senescence.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Oncogene-induced cellular senescence elicits an anti-Warburg effect.Proteomics. 2013 Sep;13(17):2542-3. doi: 10.1002/pmic.201300335. Proteomics. 2013. PMID: 23922328
References
-
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. - PubMed
-
- Hayflick L. LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Experimental Cell Research. 1965;37:614. - PubMed
-
- Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature Medicine. 2000;6:849–851. - PubMed
-
- Chiantore MV, Vannucchi S, Mangino G, Percario ZA, et al. Senescence and Cell Death Pathways and Their Role in Cancer Therapeutic Outcome. Current Medicinal Chemistry. 2009;16:287–300. - PubMed
-
- Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources