Respiratory muscle plasticity
- PMID: 23798306
- PMCID: PMC3962767
- DOI: 10.1002/cphy.c110050
Respiratory muscle plasticity
Abstract
Muscle plasticity is defined as the ability of a given muscle to alter its structural and functional properties in accordance with the environmental conditions imposed on it. As such, respiratory muscle is in a constant state of remodeling, and the basis of muscle's plasticity is its ability to change protein expression and resultant protein balance in response to varying environmental conditions. Here, we will describe the changes of respiratory muscle imposed by extrinsic changes in mechanical load, activity, and innervation. Although there is a large body of literature on the structural and functional plasticity of respiratory muscles, we are only beginning to understand the molecular-scale protein changes that contribute to protein balance. We will give an overview of key mechanisms regulating protein synthesis and protein degradation, as well as the complex interactions between them. We suggest future application of a systems biology approach that would develop a mathematical model of protein balance and greatly improve treatments in a variety of clinical settings related to maintaining both muscle mass and optimal contractile function of respiratory muscles.
© 2012 American Physiological Society. Compr Physiol 2:1441-1462, 2012.
Figures

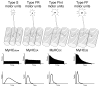


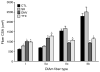
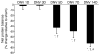


References
-
- Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol. 1995;78:1969–1976. - PubMed
-
- Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC. Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol. 2006;100:1617–1622. - PubMed
-
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

