Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human β-cardiac myosin motor function
- PMID: 23798412
- PMCID: PMC3732973
- DOI: 10.1073/pnas.1309493110
Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human β-cardiac myosin motor function
Abstract
Cardiovascular disorders are the leading cause of morbidity and mortality in the developed world, and hypertrophic cardiomyopathy (HCM) is among the most frequently occurring inherited cardiac disorders. HCM is caused by mutations in the genes encoding the fundamental force-generating machinery of the cardiac muscle, including β-cardiac myosin. Here, we present a biomechanical analysis of the HCM-causing mutation, R453C, in the context of human β-cardiac myosin. We found that this mutation causes a ∼30% decrease in the maximum ATPase of the human β-cardiac subfragment 1, the motor domain of myosin, and a similar percent decrease in the in vitro velocity. The major change in the R453C human β-cardiac subfragment 1 is a 50% increase in the intrinsic force of the motor compared with wild type, with no appreciable change in the stroke size, as observed with a dual-beam optical trap. These results predict that the overall force of the ensemble of myosin molecules in the muscle should be higher in the R453C mutant compared with wild type. Loaded in vitro motility assay confirms that the net force in the ensemble is indeed increased. Overall, this study suggests that the R453C mutation should result in a hypercontractile state in the heart muscle.
Keywords: heart disease; optical trapping; single-molecule force measurements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
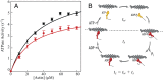
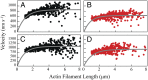
 (15). The d values are from our optical trap measurements (6 nm), and the tc values are from our actin-activated ATPase measurements. The fitting parameters are k, which represents the number of motor heads per unit length of actin filament, and ts. The maximum unloaded in vitro velocity (v0) is equal to d/ts.
(15). The d values are from our optical trap measurements (6 nm), and the tc values are from our actin-activated ATPase measurements. The fitting parameters are k, which represents the number of motor heads per unit length of actin filament, and ts. The maximum unloaded in vitro velocity (v0) is equal to d/ts.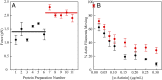
Comment in
-
Mutation that causes hypertrophic cardiomyopathy increases force production in human β-cardiac myosin.Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):12507-8. doi: 10.1073/pnas.1310669110. Epub 2013 Jul 12. Proc Natl Acad Sci U S A. 2013. PMID: 23852727 Free PMC article. No abstract available.
References
-
- Maron BJ, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–789. - PubMed
-
- Ramaraj R. Hypertrophic cardiomyopathy: Etiology, diagnosis, and treatment. Cardiol Rev. 2008;16(4):172–180. - PubMed
-
- Geisterfer-Lowrance AA, et al. A molecular basis for familial hypertrophic cardiomyopathy: A beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62(5):999–1006. - PubMed
-
- Frey N, Luedde M, Katus HA. Mechanisms of disease: Hypertrophic cardiomyopathy. Nat Rev Cardiol. 2012;9(2):91–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

