Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility
- PMID: 23798429
- PMCID: PMC3710882
- DOI: 10.1073/pnas.1300801110
Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility
Abstract
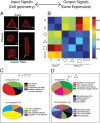
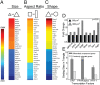
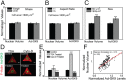
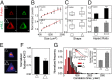
Physical forces in the form of substrate rigidity or geometrical constraints have been shown to alter gene expression profile and differentiation programs. However, the underlying mechanism of gene regulation by these mechanical cues is largely unknown. In this work, we use micropatterned substrates to alter cellular geometry (shape, aspect ratio, and size) and study the nuclear mechanotransduction to regulate gene expression. Genome-wide transcriptome analysis revealed cell geometry-dependent alterations in actin-related gene expression. Increase in cell size reinforced expression of matrix-related genes, whereas reduced cell-substrate contact resulted in up-regulation of genes involved in cellular homeostasis. We also show that large-scale changes in gene-expression profile mapped onto differential modulation of nuclear morphology, actomyosin contractility and histone acetylation. Interestingly, cytoplasmic-to-nuclear redistribution of histone deacetylase 3 modulated histone acetylation in an actomyosin-dependent manner. In addition, we show that geometric constraints altered the nuclear fraction of myocardin-related transcription factor. These fractions exhibited hindered diffusion time scale within the nucleus, correlated with enhanced serum-response element promoter activity. Furthermore, nuclear accumulation of myocardin-related transcription factor also modulated NF-κB activity. Taken together, our work provides modularity in switching gene-expression patterns by cell geometric constraints via actomyosin contractility.
Keywords: MRTF-A signaling; cell matrix interaction; chromatin remodelling; substrate geometry; transcription control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jean RP, Gray DS, Spector AA, Chen CS. Characterization of the nuclear deformation caused by changes in endothelial cell shape. J Biomech Eng. 2004;126(5):552–558. - PubMed
-
- Versaevel M, Grevesse T, Gabriele S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat Commun. 2012;3:671. - PubMed
-
- Théry M, Pépin A, Dressaire E, Chen Y, Bornens M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil Cytoskeleton. 2006;63(6):341–355. - PubMed
-
- Vergani L, Grattarola M, Nicolini C. Modifications of chromatin structure and gene expression following induced alterations of cellular shape. Int J Biochem Cell Biol. 2004;36(8):1447–1461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

