Vibrio effector protein, VopQ, forms a lysosomal gated channel that disrupts host ion homeostasis and autophagic flux
- PMID: 23798441
- PMCID: PMC3710849
- DOI: 10.1073/pnas.1307032110
Vibrio effector protein, VopQ, forms a lysosomal gated channel that disrupts host ion homeostasis and autophagic flux
Abstract
Defects in normal autophagic pathways are implicated in numerous human diseases--such as neurodegenerative diseases, cancer, and cardiomyopathy--highlighting the importance of autophagy and its proper regulation. Herein we show that Vibrio parahaemolyticus uses the type III effector VopQ (Vibrio outer protein Q) to alter autophagic flux by manipulating the partitioning of small molecules and ions in the lysosome. This effector binds to the conserved Vo domain of the vacuolar-type H(+)-ATPase and causes deacidification of the lysosomes within minutes of entering the host cell. VopQ forms a gated channel ∼18 Å in diameter that facilitates outward flux of ions across lipid bilayers. The electrostatic interactions of this type 3 secretion system effector with target membranes dictate its preference for host vacuolar-type H(+)-ATPase-containing membranes, indicating that its pore-forming activity is specific and not promiscuous. As seen with other effectors, VopQ is exploiting a eukaryotic mechanism, in this case manipulating lysosomal homeostasis and autophagic flux through transmembrane permeation.
Keywords: microbial pathogenesis; virulence; yeast vacuole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

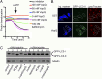

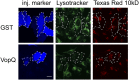
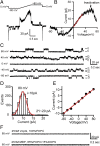
 , for specific ion k. The measured current in our recordings was expressed as the sum of sodium and chloride flows, and the estimated pore diameter is ∼18 Å. (E) The measured single-channel current–voltage relation. The linear fitting (red line) yields a single-channel conductance of 128.9 ± 3.4 pS. (F) VopQ (25 nM) incubated with 100% POPC membranes did not produce any channel activity (80 mV, n = 4). MBP (25 nM) incubated with POPC/DOPS membranes did not produce any channel activity (80 mV, n = 6).
, for specific ion k. The measured current in our recordings was expressed as the sum of sodium and chloride flows, and the estimated pore diameter is ∼18 Å. (E) The measured single-channel current–voltage relation. The linear fitting (red line) yields a single-channel conductance of 128.9 ± 3.4 pS. (F) VopQ (25 nM) incubated with 100% POPC membranes did not produce any channel activity (80 mV, n = 4). MBP (25 nM) incubated with POPC/DOPS membranes did not produce any channel activity (80 mV, n = 6).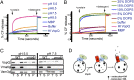
Similar articles
-
The pore-forming bacterial effector, VopQ, halts autophagic turnover.Autophagy. 2013 Dec;9(12):2169-70. doi: 10.4161/auto.26449. Epub 2013 Oct 8. Autophagy. 2013. PMID: 24145145 Free PMC article.
-
Vibrio effector protein VopQ inhibits fusion of V-ATPase-containing membranes.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):100-5. doi: 10.1073/pnas.1413764111. Epub 2014 Dec 1. Proc Natl Acad Sci U S A. 2015. PMID: 25453092 Free PMC article.
-
A distinct inhibitory mechanism of the V-ATPase by Vibrio VopQ revealed by cryo-EM.Nat Struct Mol Biol. 2020 Jun;27(6):589-597. doi: 10.1038/s41594-020-0429-1. Epub 2020 May 18. Nat Struct Mol Biol. 2020. PMID: 32424347
-
Lysosomal physiology.Annu Rev Physiol. 2015;77:57-80. doi: 10.1146/annurev-physiol-021014-071649. Annu Rev Physiol. 2015. PMID: 25668017 Free PMC article. Review.
-
Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular.Int J Mol Sci. 2020 Apr 8;21(7):2576. doi: 10.3390/ijms21072576. Int J Mol Sci. 2020. PMID: 32276321 Free PMC article. Review.
Cited by
-
Unveiling potential virulence determinants in Vibrio isolates from Anadara tuberculosa through whole genome analyses.Microbiol Spectr. 2024 Feb 6;12(2):e0292823. doi: 10.1128/spectrum.02928-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189292 Free PMC article.
-
Electroporation of functional bacterial effectors into mammalian cells.J Vis Exp. 2015 Jan 19;(95):52296. doi: 10.3791/52296. J Vis Exp. 2015. PMID: 25650771 Free PMC article.
-
Vibrio deploys type 2 secreted lipase to esterify cholesterol with host fatty acids and mediate cell egress.Elife. 2020 Aug 18;9:e58057. doi: 10.7554/eLife.58057. Elife. 2020. PMID: 32808593 Free PMC article.
-
Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication.Cell Death Dis. 2015 May 21;6(5):e1770. doi: 10.1038/cddis.2015.136. Cell Death Dis. 2015. PMID: 25996297 Free PMC article.
-
Secretory granule protein chromogranin B (CHGB) forms an anion channel in membranes.Life Sci Alliance. 2018 Sep 24;1(5):e201800139. doi: 10.26508/lsa.201800139. eCollection 2018 Oct. Life Sci Alliance. 2018. PMID: 30456382 Free PMC article.
References
-
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27(6):421–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

