The impact of protein-conjugate polysaccharide vaccines: an endgame for meningitis?
- PMID: 23798695
- PMCID: PMC3720045
- DOI: 10.1098/rstb.2012.0147
The impact of protein-conjugate polysaccharide vaccines: an endgame for meningitis?
Abstract
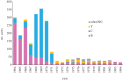
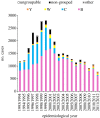
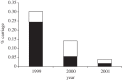
The development and implementation of conjugate polysaccharide vaccines against invasive bacterial diseases, specifically those caused by the encapsulated bacteria Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumoniae, has been one of the most effective public health innovations of the last 25 years. These vaccines have resulted in significant reductions in childhood morbidity and mortality worldwide, with their effectiveness due in large part to their ability to induce long-lasting immunity in a range of age groups. At the population level this immunity reduces carriage and interrupts transmission resulting in herd immunity; however, these beneficial effects can be counterbalanced by the selection pressures that immunity against carriage can impose, potentially promoting the emergence and spread of virulent vaccine escape variants. Studies following the implementation of meningococcal serogroup C vaccines improved our understanding of these effects in relation to the biology of accidental pathogens such as the meningococcus. This understanding has enabled the refinement of the implementation of conjugate polysaccharide vaccines against meningitis-associated bacteria, and will be crucial in maintaining and improving vaccine control of these infections. To date there is little evidence for the spread of virulent vaccine escape variants of the meningococcus and H. influenzae, although this has been reported in pneumococci.
Keywords: Haemophilus influenzae; Neisseria meningitidis; Streptococcus pneumoniae; herd immunity; population biology; vaccines.
Figures
References
-
- Pollard AJ, Perrett KP, Beverley PC. 2009. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9, 212–22010.1038/nri2494 (doi:10.1038/nri2494) - DOI - DOI - PubMed
-
- Gendelman HE, Persidsky Y. 2005. Infections of the nervous system. Lancet Neurol. 4, 12–1310.1016/S1474-4422(04)00951-2 (doi:10.1016/S1474-4422(04)00951-2) - DOI - DOI - PMC - PubMed
-
- Bamberger DM. 2010. Diagnosis, initial management, and prevention of meningitis. Am. Fam. Physician 82, 1491–1498 - PubMed
-
- McIntyre PB, O'Brien KL, Greenwood B, van de Beek D. 2012. Effect of vaccines on bacterial meningitis worldwide. Lancet 380, 1703–171110.1016/S0140-6736(12)61187-8 (doi:10.1016/S0140-6736(12)61187-8) - DOI - DOI - PubMed
-
- Schneerson R, Barrera O, Sutton A, Robbins JB. 1980. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide–protein conjugates. J. Exp. Med. 152, 361–37610.1084/jem.152.2.361 (doi:10.1084/jem.152.2.361) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
