Performance of serum biomarkers for the early detection of invasive aspergillosis in febrile, neutropenic patients: a multi-state model
- PMID: 23799048
- PMCID: PMC3683047
- DOI: 10.1371/journal.pone.0065776
Performance of serum biomarkers for the early detection of invasive aspergillosis in febrile, neutropenic patients: a multi-state model
Abstract
Background: The performance of serum biomarkers for the early detection of invasive aspergillosis expectedly depends on the timing of test results relative to the empirical administration of antifungal therapy during neutropenia, although a dynamic evaluation framework is lacking.
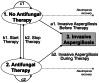
Methods: We developed a multi-state model describing simultaneously the likelihood of empirical antifungal therapy and the risk of invasive aspergillosis during neutropenia. We evaluated whether the first positive test result with a biomarker is an independent predictor of invasive aspergillosis when both diagnostic information used to treat and risk factors of developing invasive aspergillosis are taken into account over time. We applied the multi-state model to a homogeneous cohort of 185 high-risk patients with acute myeloid leukemia. Patients were prospectively screened for galactomannan antigenemia twice a week for immediate treatment decision; 2,214 serum samples were collected on the same days and blindly assessed for (1->3)- β-D-glucan antigenemia and a quantitative PCR assay targeting a mitochondrial locus.
Results: The usual evaluation framework of biomarker performance was unable to distinguish clinical benefits of β-glucan or PCR assays. The multi-state model evidenced that the risk of invasive aspergillosis is a complex time function of neutropenia duration and risk management. The quantitative PCR assay accelerated the early detection of invasive aspergillosis (P = .010), independently of other diagnostic information used to treat, while β-glucan assay did not (P = .53).
Conclusions: The performance of serum biomarkers for the early detection of invasive aspergillosis is better apprehended by the evaluation of time-varying predictors in a multi-state model. Our results provide strong rationale for prospective studies testing a preemptive antifungal therapy, guided by clinical, radiological, and bi-weekly blood screening with galactomannan antigenemia and a standardized quantitative PCR assay.
Conflict of interest statement
Figures
References
-
- Segal BH (2009) Aspergillosis. N Engl J Med 360: 1870–1884. - PubMed
-
- Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, et al. (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34: 7–14. - PubMed
-
- de Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, et al. (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46: 1813–1821. - PMC - PubMed
-
- Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, et al. (1984) Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med 100: 345–351. - PubMed
-
- Muhlemann K, Wenger C, Zenhausern R, Tauber MG (2005) Risk factors for invasive aspergillosis in neutropenic patients with hematologic malignancies. Leukemia 19: 545–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources