Evaluation of small intestine grafts decellularization methods for corneal tissue engineering
- PMID: 23799114
- PMCID: PMC3682956
- DOI: 10.1371/journal.pone.0066538
Evaluation of small intestine grafts decellularization methods for corneal tissue engineering
Abstract
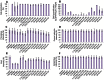
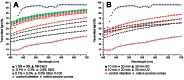
Advances in the development of cornea substitutes by tissue engineering techniques have focused on the use of decellularized tissue scaffolds. In this work, we evaluated different chemical and physical decellularization methods on small intestine tissues to determine the most appropriate decellularization protocols for corneal applications. Our results revealed that the most efficient decellularization agents were the SDS and triton X-100 detergents, which were able to efficiently remove most cell nuclei and residual DNA. Histological and histochemical analyses revealed that collagen fibers were preserved upon decellularization with triton X-100, NaCl and sonication, whereas reticular fibers were properly preserved by decellularization with UV exposure. Extracellular matrix glycoproteins were preserved after decellularization with SDS, triton X-100 and sonication, whereas proteoglycans were not affected by any of the decellularization protocols. Tissue transparency was significantly higher than control non-decellularized tissues for all protocols, although the best light transmittance results were found in tissues decellularized with SDS and triton X-100. In conclusion, our results suggest that decellularized intestinal grafts could be used as biological scaffolds for cornea tissue engineering. Decellularization with triton X-100 was able to efficiently remove all cells from the tissues while preserving tissue structure and most fibrillar and non-fibrillar extracellular matrix components, suggesting that this specific decellularization agent could be safely used for efficient decellularization of SI tissues for cornea TE applications.
Conflict of interest statement
Figures






References
-
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920–926. - PubMed
-
- Alaminos M, Del Carmen Sanchez-Quevedo M, Munoz-Avila JI, Serrano D, Medialdea S, et al. (2006) Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest Ophthalmol Vis Sci 47: 3311–3317. - PubMed
-
- Ionescu AM, de la Cruz Cardona J, Gonzalez-Andrades M, Alaminos M, Campos A, et al. (2010) UV absorbance of a bioengineered corneal stroma substitute in the 240–400 nm range. Cornea 29: 895–898 doi: 10.1097/ICO.0b013e3181ca3650 - DOI - PubMed
-
- Ionescu AM, Alaminos M, de la Cruz Cardona J, de Dios Garcia-Lopez Duran J, Gonzalez-Andrades M, et al. (2011) Investigating a novel nanostructured fibrin-agarose biomaterial for human cornea tissue engineering: rheological properties. J Mech Behav Biomed Mater 4: 1963–1973 doi: 10.1016/j.jmbbm.2011.06.013 - DOI - PubMed
-
- Cardona Jde L, Ionescu AM, Gomez-Sotomayor R, Gonzalez-Andrades M, Campos A, et al. (2011) Transparency in a fibrin and fibrin-agarose corneal stroma substitute generated by tissue engineering. Cornea 30: 1428–1435 doi: 10.1097/ICO.0b013e31821bdfd4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

