Selaginellatamariscina attenuates metastasis via Akt pathways in oral cancer cells
- PMID: 23799155
- PMCID: PMC3683067
- DOI: 10.1371/journal.pone.0068035
Selaginellatamariscina attenuates metastasis via Akt pathways in oral cancer cells
Abstract
Background: Crude extracts of Selaginellatamariscina, an oriental medicinal herb, have been evidenced to treat several human diseases. This study investigated the mechanisms by which Selaginellatamariscina inhibits the invasiveness of human oral squamous-cell carcinoma (OSCC) HSC-3 cells.
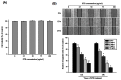
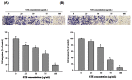
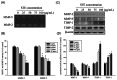
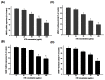
Methodology/principal findings: Herein, we demonstrate that Selaginellatamariscina attenuated HSC-3 cell migration and invasion in a dose-dependent manner. The anti-metastatic activities of Selaginellatamariscina occurred at least partially because of the down-regulation of matrix metalloproteinases (MMP)-2 and MMP-9 gelatinase activity and the down-regulation of protein expression. The expression and function of both MMP-2 and MMP-9 were regulated by Selaginellatamariscina at a transcriptional level, as shown by quantitative real-time PCR and reporter assays. Chromatin immunoprecipitation (ChIP) data further indicated that binding of the cAMP response element-binding (CREB) protein and activating protein-1 (AP-1) to the MMP-2 promoter diminished at the highest dosage level of Selaginellatamariscina. The DNA-binding activity of specificity protein 1 (SP-1) to the MMP-9 promoter was also suppressed at the same concentration. Selaginellatamariscina did not affect the mitogen-activated protein kinase signaling pathway, but did inhibit the effects of gelatinase by reducing the activation of serine-threonine kinase Akt.
Conclusions: These results demonstrate that Selaginellatamariscina may be a potent adjuvant therapeutic agent in the prevention of oral cancer.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

