Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds
- PMID: 23799306
- PMCID: PMC3856931
- DOI: 10.1089/ten.TEA.2013.0256
Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds
Abstract
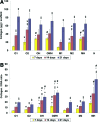
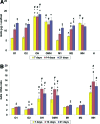
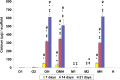
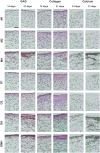
In this work, human mesenchymal stem cells (hMSCs) and their osteogenically precultured derivatives were directly cocultured with human umbilical vein endothelial cells (HUVECs) on electrospun three-dimensional poly(ɛ-caprolactone) microfiber scaffolds to evaluate the coculture's effect on the generation of osteogenic constructs. Specifically, cells were cultured on scaffolds for up to 3 weeks, and the cellularity, alkaline phosphatase (ALP) activity, and bone-like matrix formation were assessed. Constructs with cocultures and monocultures had almost identical cellularity after the first week, however, lower cellularity was observed in cocultures compared to monocultures during the subsequent 2 weeks of culture. Scaffolds with cocultures showed a significantly higher ALP activity, glycosaminoglycan and collagen production, as well as greater calcium deposition over the course of study compared to monocultures of hMSCs. Furthermore, the osteogenic outcome was equally robust in cocultures containing osteogenically precultured and non-precultured hMSCs. The results demonstrate that the combination of MSC and HUVEC populations within a porous scaffold material under osteogenic culture conditions is an effective strategy to promote osteogenesis.
Figures




Similar articles
-
Flow perfusion co-culture of human mesenchymal stem cells and endothelial cells on biodegradable polymer scaffolds.Ann Biomed Eng. 2014 Jul;42(7):1381-90. doi: 10.1007/s10439-013-0862-y. Epub 2013 Jul 11. Ann Biomed Eng. 2014. PMID: 23842695 Free PMC article.
-
Effects of Fiber Alignment and Coculture with Endothelial Cells on Osteogenic Differentiation of Mesenchymal Stromal Cells.Tissue Eng Part C Methods. 2020 Jan;26(1):11-22. doi: 10.1089/ten.TEC.2019.0232. Epub 2019 Dec 27. Tissue Eng Part C Methods. 2020. PMID: 31774033
-
Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting.Biofabrication. 2020 Feb 19;12(2):025013. doi: 10.1088/1758-5090/ab6a1d. Biofabrication. 2020. PMID: 31929117
-
Adipose tissue-derived mesenchymal stem cells as monocultures or cocultures with human umbilical vein endothelial cells: performance in vitro and in rat cranial defects.J Biomed Mater Res A. 2014 Apr;102(4):1026-36. doi: 10.1002/jbm.a.34775. Epub 2013 Jun 4. J Biomed Mater Res A. 2014. PMID: 23640784
-
Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:479-490. doi: 10.1016/j.msec.2019.01.127. Epub 2019 Jan 30. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889723 Free PMC article.
Cited by
-
Flow perfusion co-culture of human mesenchymal stem cells and endothelial cells on biodegradable polymer scaffolds.Ann Biomed Eng. 2014 Jul;42(7):1381-90. doi: 10.1007/s10439-013-0862-y. Epub 2013 Jul 11. Ann Biomed Eng. 2014. PMID: 23842695 Free PMC article.
-
3D tissue-engineered model of Ewing's sarcoma.Adv Drug Deliv Rev. 2014 Dec 15;79-80:155-71. doi: 10.1016/j.addr.2014.07.012. Epub 2014 Aug 7. Adv Drug Deliv Rev. 2014. PMID: 25109853 Free PMC article. Review.
-
Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo.Ann N Y Acad Sci. 2017 Dec;1409(1):3-17. doi: 10.1111/nyas.13451. Epub 2017 Oct 6. Ann N Y Acad Sci. 2017. PMID: 28984359 Free PMC article. Review.
-
Angiogenic and Osteogenic Synergy of Human Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells Cocultured on a Nanomatrix.Sci Rep. 2018 Oct 24;8(1):15749. doi: 10.1038/s41598-018-34033-2. Sci Rep. 2018. PMID: 30356078 Free PMC article.
-
Multi-compartment scaffold fabricated via 3D-printing as in vitro co-culture osteogenic model.Sci Rep. 2018 Oct 11;8(1):15130. doi: 10.1038/s41598-018-33472-1. Sci Rep. 2018. PMID: 30310164 Free PMC article.
References
-
- Bauer T.W. Muschler G.F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10. - PubMed
-
- Rouwkema J. Rivron N.C. van Blitterswijk C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434. - PubMed
-
- Kanczler J.M. Oreffo R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

