DNA polymerase κ-dependent DNA synthesis at stalled replication forks is important for CHK1 activation
- PMID: 23799366
- PMCID: PMC3730229
- DOI: 10.1038/emboj.2013.148
DNA polymerase κ-dependent DNA synthesis at stalled replication forks is important for CHK1 activation
Abstract
Formation of primed single-stranded DNA at stalled replication forks triggers activation of the replication checkpoint signalling cascade resulting in the ATR-mediated phosphorylation of the Chk1 protein kinase, thus preventing genomic instability. By using siRNA-mediated depletion in human cells and immunodepletion and reconstitution experiments in Xenopus egg extracts, we report that the Y-family translesion (TLS) DNA polymerase kappa (Pol κ) contributes to the replication checkpoint response and is required for recovery after replication stress. We found that Pol κ is implicated in the synthesis of short DNA intermediates at stalled forks, facilitating the recruitment of the 9-1-1 checkpoint clamp. Furthermore, we show that Pol κ interacts with the Rad9 subunit of the 9-1-1 complex. Finally, we show that this novel checkpoint function of Pol κ is required for the maintenance of genomic stability and cell proliferation in unstressed human cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
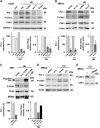


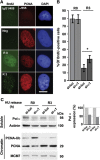

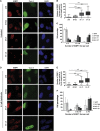
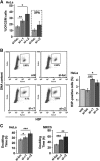
Comment in
-
Pol κ in replication checkpoint.Cell Cycle. 2013 Dec 15;12(24):3713-4. doi: 10.4161/cc.26976. Epub 2013 Oct 28. Cell Cycle. 2013. PMID: 24189533 Free PMC article. No abstract available.
References
-
- Avkin S, Goldsmith M, Velasco-Miguel S, Geacintov N, Friedberg EC, Livneh Z (2004) Quantitative analysis of translesion DNA synthesis across a benzo[a]pyrene-guanine adduct in mammalian cells: the role of DNA polymerase kappa. J Biol Chem 279: 53298–53305 - PubMed
-
- Bi X, Slater DM, Ohmori H, Vaziri C (2005) DNA polymerase kappa is specifically required for recovery from the benzo[a]pyrene-dihydrodiol epoxide (BPDE)-induced S-phase checkpoint. J Biol Chem 280: 22343–22355 - PubMed
-
- Biard DS, Despras E, Sarasin A, Angulo JF (2005) Development of new EBV-based vectors for stable expression of small interfering RNA to mimick human syndromes: application to NER gene silencing. Mol Cancer Res 3: 519–529 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

