Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics
- PMID: 23799535
- PMCID: PMC3734658
- DOI: 10.1038/mt.2013.124
Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics
Abstract
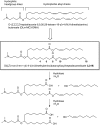
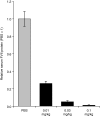
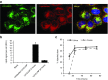
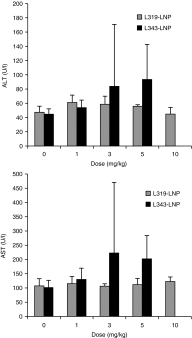
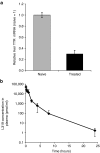
In recent years, RNA interference (RNAi) therapeutics, most notably with lipid nanoparticle-based delivery systems, have advanced into human clinical trials. The results from these early clinical trials suggest that lipid nanoparticles (LNPs), and the novel ionizable lipids that comprise them, will be important materials in this emerging field of medicine. A persistent theme in the use of materials for biomedical applications has been the incorporation of biodegradability as a means to improve biocompatibility and/or to facilitate elimination. Therefore, the aim of this work was to further advance the LNP platform through the development of novel, next-generation lipids that combine the excellent potency of the most advanced lipids currently available with biodegradable functionality. As a representative example of this novel class of biodegradable lipids, the lipid evaluated in this work displays rapid elimination from plasma and tissues, substantially improved tolerability in preclinical studies, while maintaining in vivo potency on par with that of the most advanced lipids currently available.
Figures

References
-
- Dose escalation trial to evaluate the safety tolerability pharmacokinetics and pharmacodynamics of intravenous ALN-VSP02 In patients with advanced solid tumors with liver involvement.Registry of Federally and Privately Supported Clinical Trials, U.S. National Institutes of Health. < . < http://clinicaltrials.gov/ct2/show/NCT00882180 >.
-
- Gollob J.2011Phase I Dose Escalation Study of ALN-VSP02, a novel RNAi therapeutic for solid tumors with liver involvement.ASCO Annual Meeting, 48 June, Chicago, USA
-
- Trial to Evaluate Safety and Tolerability of ALN-TTR01 in Transthyretin (TTR) Amyloidosis Registry of Federally and Privately Supported Clinical Trials, U.S. National Institutes of Health . < http://clinicaltrials.gov/ct2/show/NCT01148953 >.
-
- Coelho T.2012Final Phase I Safety, Pharmacokinetic and Pharmacodynamic Results for ALN-TTR01, a novel rnai therapeutic for the treatment of transthyretin amyloidosis.XIII International Symposium on Amyloidosis, 10 May 2012, Groningen, Netherlands
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

