The endocrine pancreas: insights into development, differentiation, and diabetes
- PMID: 23799564
- PMCID: PMC3420142
- DOI: 10.1002/wdev.44
The endocrine pancreas: insights into development, differentiation, and diabetes
Abstract
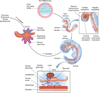
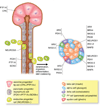
In the developing embryo, appropriate patterning of the endoderm fated to become pancreas requires the spatial and temporal coordination of soluble factors secreted by the surrounding tissues. Once pancreatic progenitor cells are specified in the developing gut tube epithelium, epithelial-mesenchymal interactions, as well as a cascade of transcription factors, subsequently delineate three distinct lineages, including endocrine, exocrine, and ductal cells. Simultaneous morphological changes, including branching, vascularization, and proximal organ development, also influence the process of specification and differentiation. Decades of research using mouse genetics have uncovered many of the key factors involved in pancreatic cell fate decisions. When pancreas development or islet cell functions go awry, due to mutations in genes important for proper organogenesis and development, the result can lead to a common pancreatic affliction, diabetes mellitus. Current treatments for diabetes are adequate but not curative. Therefore, researchers are utilizing the current understanding of normal embryonic pancreas development in vivo, to direct embryonic stem cells toward a pancreatic fate with the goal of transplanting these in vitro generated 'islets' into patients. Mimicking development in vitro has proven difficult; however, significant progress has been made and the current differentiation protocols are becoming more efficient. The continued partnership between developmental biologists and stem cell researchers will guarantee that the in vitro generation of insulin-producing β cells is a possible therapeutic option for the treatment of diabetes.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Murtaugh LC, Leach SD. A case of mistaken identity? Nonductal origins of pancreatic "ductal" cancers. Cancer Cell. 2007;11:211–213. - PubMed
-
- Oberg K. Pancreatic endocrine tumors. Semin Oncol. 2010;37:594–618. - PubMed
-
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

