Control of adult stem cells in vivo by a dynamic physiological environment: diet-dependent systemic factors in Drosophila and beyond
- PMID: 23799567
- PMCID: PMC3733242
- DOI: 10.1002/wdev.48
Control of adult stem cells in vivo by a dynamic physiological environment: diet-dependent systemic factors in Drosophila and beyond
Abstract
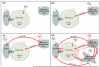
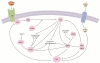
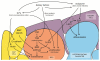
Adult stem cells are inextricably linked to whole-body physiology and nutrient availability through complex systemic signaling networks. A full understanding of how stem cells sense and respond to dietary fluctuations will require identifying key systemic mediators, as well as elucidating how they are regulated and integrated with local and intrinsic factors across multiple tissues. Studies focused on the Drosophila germline have generated valuable insights into how stem cells are controlled by diet-dependent pathways, and increasing evidence suggests that diverse adult stem cell populations respond to nutrients through similar mechanisms. Systemic signals, including nutrients themselves and diet-regulated hormones such as Insulin/Insulin-like growth factor or steroid hormones, can directly or indirectly affect stem cell behavior by modifying local cell-cell communication or intrinsic factors. The physiological regulation of stem cells in response to nutritional status not only is a fascinating biological problem, but also has clinical implications, as research in this field holds the key to noninvasive approaches for manipulating stem cells in vivo. In addition, given the known associations between diet, stem cells, and cancer risk, this research may inspire novel anticancer therapies.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Martin-Salces M, de Paz R, Canales MA, Mesejo A, Hernandez-Navarro F. Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition. 2008;24:769–775. - PubMed
-
- Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61–68. - PubMed
-
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. - PubMed
Further Reading/Resources
-
- [accessed August 29, 2011];StemBook: An open-access collection of peer-reviewed chapters covering topics related to stem cell biology. maintained by the Harvard Stem Cell Institute. http://www.stembook.org/
-
- [accessed August 29, 2011];Society for Developmental Biology, Interactive Fly: An on-line guide to Drosophila development, including a detailed atlas of Drosophila development and a tutorial on oogenesis and spermatogenesis. http://www.sdbonline.org/fly/aimain/1aahome.htm.
-
- National Human Genome Research Institute model organism ENCyclopedia Of DNA Elements [accessed August 29, 2011];Open-access database of genomic datasets from Drosophila and C. elegans, assembled by a consortium of 11 primary projects, identifying transcription factor binding sites, histone modifications, chromatin structure, and other sequence-based functional elements. modENCODE. http://www.modencode.org/
-
- [accessed August 29, 2011];Mouse Phenotype Database: Comprehensive database containing phenotypic characteristics of commonly used inbred mouse strains. maintained by The Jackson Laboratory. http://phenome.jax.org/
-
- Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

