Heterogeneity in nanoparticles influences biodistribution and targeting
- PMID: 23799984
- PMCID: PMC3883796
- DOI: 10.2217/nnm.13.70
Heterogeneity in nanoparticles influences biodistribution and targeting
Abstract
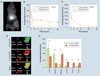
Aim: A large fraction of the administered dose of nanoparticles (NPs) localizes into nontarget tissue, which could be due to the heterogeneous population of NPs.
Materials & methods: To investigate the impact of the above issue, we simultaneously tracked the biodistribution using optical imaging of two different sized poly(d,l-lactide co-glycolide) NPs, which also varied in their surface charge and texture, in a prostate tumor xenograft mouse model.
Results: Although formulated using the same polymer and emulsifier concentration, small NPs were neutral (S-neutral-NPs), whereas large NPs were anionic (L-anionic-NPs). Simultaneous injection of these NPs, representing heterogeneity, shows significantly different biodistribution. S-neutral-NPs demonstrated longer circulation time than L-anionic-NPs (t1/2 = 96 vs 13 min); accounted for 75% of total NPs accumulated in the tumor; and showed 13-fold greater tumor to liver signal intensity ratio than L-anionic-NPs.
Conclusion: The data underscore the importance of formulating nanocarriers of specific properties to enhance their targeting efficacy.
Figures



References
-
- Avgoustakis K, Beletsi A, Panagi Z, et al. Effect of copolymer composition on the physicochemical characteristics, in vitro stability, and biodistribution of PLGA-mPEG nanoparticles. Int. J. Pharm. 2003;259(1–2):115–127. - PubMed
-
- Litzinger DC, Buiting AMJ, Van Rooijen N, Huang L. Effect of liposome size on the circulation time and intraorgandistributionof amphipathic poly(ethylene glycol)-containing liposomes. Biochim. Biophys. Acta. 1994;1190(1):99–107. - PubMed
-
- Rao DA, Robinson JR. Effect of size, surface properties of biodegradable PLGA-PMA: PLA:PEG nanoparticles on lymphatic uptake and retention in rats. J. Control. Release. 2008;132(3):e45–e47.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
