Macromolecular juggling by ubiquitylation enzymes
- PMID: 23800009
- PMCID: PMC3748819
- DOI: 10.1186/1741-7007-11-65
Macromolecular juggling by ubiquitylation enzymes
Abstract
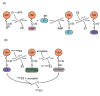
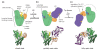
The posttranslational modification of target proteins with ubiquitin and ubiquitin-like proteins is accomplished by the sequential action of E1, E2, and E3 enzymes. Members of the E1 and E3 enzyme families can undergo particularly large conformational changes during their catalytic cycles, involving the remodeling of domain interfaces. This enables the efficient, directed and regulated handover of ubiquitin from one carrier to the next one. We review some of these conformational transformations, as revealed by crystallographic studies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

