Three-dimensional characterization of tissue-engineered constructs by contrast-enhanced nanofocus computed tomography
- PMID: 23800097
- PMCID: PMC3936499
- DOI: 10.1089/ten.TEC.2013.0041
Three-dimensional characterization of tissue-engineered constructs by contrast-enhanced nanofocus computed tomography
Abstract
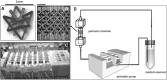
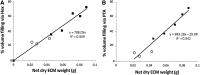
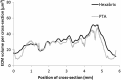
To successfully implement tissue-engineered (TE) constructs as part of a clinical therapy, it is necessary to develop quality control tools that will ensure accurate and consistent TE construct release specifications. Hence, advanced methods to monitor TE construct properties need to be further developed. In this study, we showed proof of concept for contrast-enhanced nanofocus computed tomography (CE-nano-CT) as a whole-construct imaging technique with a noninvasive potential that enables three-dimensional (3D) visualization and quantification of in vitro engineered extracellular matrix (ECM) in TE constructs. In particular, we performed a 3D qualitative and quantitative structural and spatial assessment of the in vitro engineered ECM, formed during static and perfusion bioreactor cell culture in 3D TE scaffolds, using two contrast agents, namely, Hexabrix® and phosphotungstic acid (PTA). To evaluate the potential of CE-nano-CT, a comparison was made to standardly used techniques such as Live/Dead viability/cytotoxicity, Picrosirius Red staining, and to net dry weight measurements of the TE constructs. When using Hexabrix as the contrast agent, the ECM volume fitted linearly with the net dry ECM weight independent from the flow rate used, thus suggesting that it stains most of the ECM. When using PTA as the contrast agent, comparing to net weight measurements showed that PTA only stains a part of the ECM. This was attributed to the binding specificity of this contrast agent. In addition, the PTA-stained CE-nano-CT data showed pronounced distinction between flow conditions when compared to Hexabrix, indicating culture-specific structural ECM differences. This novel type of information can contribute to optimize bioreactor culture conditions and potentially critical quality characteristics of TE constructs such as ECM quantity and homogeneity, facilitating the gradual transformation of TE constructs in well-characterized TE products.
Figures





References
-
- Eniwumide J.O., Yuan H., Cartmell S.H., Meijer G.J., and de Bruijn J.D.Ectopic bone formation in bone marrow stem cell seeded calcium phosphate scaffolds as compared to autograft and (cell seeded) allograft. Eur Cell Mater 14,30.; discussion 9.2007 - PubMed
-
- Hedberg E.L., Kroese-Deutman H.C., Shih C.K., Lemoine J.J., Liebschner M.A.K., Miller M.J., et al. . Methods: a comparative analysis of radiography, microcomputed tomography, and histology for bone tissue engineering. Tissue Eng 11,1356, 2005 - PubMed
-
- Stephens J.S., Cooper J.A., Phelan F.R., and Dunkers J.P.Perfusion flow bioreactor for 3D in situ imaging: investigating cell/biomaterials interactions. Biotechnol Bioeng 97,952, 2007 - PubMed
-
- Smith L.E., Smallwood R., and Macneil S.A comparison of imaging methodologies for 3D tissue engineering. Microsc Res Tech 73,1123, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

