Disuse-induced muscle wasting
- PMID: 23800384
- PMCID: PMC3856924
- DOI: 10.1016/j.biocel.2013.06.011
Disuse-induced muscle wasting
Abstract
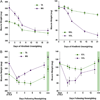
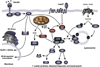
Loss of skeletal muscle mass occurs frequently in clinical settings in response to joint immobilization and bed rest, and is induced by a combination of unloading and inactivity. Disuse-induced atrophy will likely affect every person in his or her lifetime, and can be debilitating especially in the elderly. Currently there are no good therapies to treat disuse-induced muscle atrophy, in part, due to a lack of understanding of the cellular and molecular mechanisms responsible for the induction and maintenance of muscle atrophy. Our current understanding of disuse atrophy comes from the investigation of a variety of models (joint immobilization, hindlimb unloading, bed rest, spinal cord injury) in both animals and humans. Under conditions of unloading, it is widely accepted that there is a decrease in protein synthesis, however, the role of protein degradation, especially in humans, is debated. This review will examine the current understanding of the molecular and cellular mechanisms regulating muscle loss under disuse conditions, discussing the similarities and areas of dispute between the animal and human literature. This article is part of a Directed Issue entitled: Molecular basis of muscle wasting.
Keywords: Protein degradation; Protein synthesis; Reloading; Ubiquitin ligases; Unloading.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
-
- Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. Journal of Applied Physiology. 2003;95:2185–2201. - PubMed
-
- Andrianjafiniony T, Dupre-Aucouturier S, Letexier D, Couchoux H, Desplanches D. Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. American Journal of Physiology – Cell Physiology. 2010;299:C307–C315. - PubMed
-
- Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, et al. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. Journal of Applied Physiology. 1998;84:157–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

