Revisiting clinical trials using EGFR inhibitor-based regimens in patients with advanced non-small cell lung cancer: a retrospective analysis of an MD Anderson Cancer Center phase I population
- PMID: 23800712
- PMCID: PMC3742837
- DOI: 10.18632/oncotarget.1028
Revisiting clinical trials using EGFR inhibitor-based regimens in patients with advanced non-small cell lung cancer: a retrospective analysis of an MD Anderson Cancer Center phase I population
Abstract
Purpose: Single-agent EGFR inhibitor therapy is effective mainly in patients with lung cancer and EGFR mutations. Treating patients who develop resistance, or who are insensitive from the outset, often because of resistant mutations, other aberrations or the lack of an EGFR mutation, probably requires rational combinations. We therefore investigated the outcome of EGFR inhibitor-based combination regimens in patients with heavily-pretreated non-small cell lung cancer (NSCLC) referred to a Phase I Clinic.
Methods: We reviewed the electronic records of patients with NSCLC treated with an EGFR inhibitor-based combination regimen: erlotinib and cetuximab; erlotinib, cetuximab and bevacizumab; erlotinib and dasatinib; erlotinib and bortezomib; or cetuximab and sirolimus.
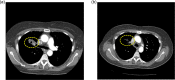
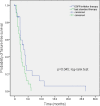
Results: EGFR mutations were detected in 16% of patients (21/131). EGFR inhibitor-based combination regimens were administered to 15 patients with EGFR-mutant NSCLC and 24 with EGFR wild-type disease. Stable disease (SD) ≥6 months/partial remission (PR) was attained in 20% of EGFR-mutant patients (3/15; two with sensitive mutations and secondary resistance to prior erlotinib, and one with a resistant mutation), as well as 26% of evaluable patients (5/19) with wild-type disease. One of three evaluable patients with squamous cell histology achieved SD for 26.5 months (EGFR wild-type, TP53-mutant, regimen=erlotinib, cetuximab and bevacizumab).
Conclusions: Eight of 34 evaluable patients (24%) with advanced, refractory NSCLC evaluable for response achieved SD ≥6 months/PR (PR=3; SD ≥6 months=5) on EGFR inhibitor-based combination regimens (erlotinib, cetuximab; erlotinib, cetuximab and bevacizumab; and, erlotinib, bortezomib), including patients with secondary resistance to single-agent EGFR inhibitors, resistant mutations, wild-type disease, and, squamous histology.
Figures

References
-
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. - PubMed
-
- Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12(3 Pt 1):839–844. - PubMed
-
- Janku F, Garrido-Laguna I, Petruzelka LB, Stewart DJ, Kurzrock R. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1601–1612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

