Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice
- PMID: 23800963
- PMCID: PMC3723616
- DOI: 10.1105/tpc.113.113068
Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice
Abstract
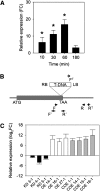
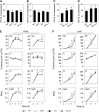
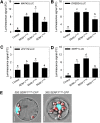
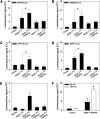
Early detection of salt stress is vital for plant survival and growth. Still, the molecular processes controlling early salt stress perception and signaling are not fully understood. Here, we identified salt-responsive ERF1 (SERF1), a rice (Oryza sativa) transcription factor (TF) gene that shows a root-specific induction upon salt and hydrogen peroxide (H2O2) treatment. Loss of SERF1 impairs the salt-inducible expression of genes encoding members of a mitogen-activated protein kinase (MAPK) cascade and salt tolerance-mediating TFs. Furthermore, we show that SERF1-dependent genes are H2O2 responsive and demonstrate that SERF1 binds to the promoters of MAPK kinase kinase6 (MAP3K6), MAPK5, dehydration-responsive element bindinG2A (DREB2A), and zinc finger protein179 (ZFP179) in vitro and in vivo. SERF1 also directly induces its own gene expression. In addition, SERF1 is a phosphorylation target of MAPK5, resulting in enhanced transcriptional activity of SERF1 toward its direct target genes. In agreement, plants deficient for SERF1 are more sensitive to salt stress compared with the wild type, while constitutive overexpression of SERF1 improves salinity tolerance. We propose that SERF1 amplifies the reactive oxygen species-activated MAPK cascade signal during the initial phase of salt stress and translates the salt-induced signal into an appropriate expressional response resulting in salt tolerance.
Figures



Similar articles
-
The contribution of SERF1 to root-to-shoot signaling during salinity stress in rice.Plant Signal Behav. 2014;9(1):e27540. doi: 10.4161/psb.27540. Epub 2014 Jan 21. Plant Signal Behav. 2014. PMID: 24451326 Free PMC article.
-
Marker-free transgenic rice plant overexpressing pea LecRLK imparts salinity tolerance by inhibiting sodium accumulation.Plant Mol Biol. 2019 Feb;99(3):265-281. doi: 10.1007/s11103-018-0816-8. Epub 2019 Jan 2. Plant Mol Biol. 2019. PMID: 30604324
-
Functional analysis of a novel Cys2/His2-type zinc finger protein involved in salt tolerance in rice.J Exp Bot. 2010 Jun;61(10):2807-18. doi: 10.1093/jxb/erq120. Epub 2010 May 11. J Exp Bot. 2010. PMID: 20460361 Free PMC article.
-
Wheat TaTIP4;1 Confers Enhanced Tolerance to Drought, Salt and Osmotic Stress in Arabidopsis and Rice.Int J Mol Sci. 2022 Feb 14;23(4):2085. doi: 10.3390/ijms23042085. Int J Mol Sci. 2022. PMID: 35216200 Free PMC article.
-
Deciphering molecular regulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) signalling networks in Oryza genus amid environmental stress.Plant Cell Rep. 2024 Jul 1;43(7):185. doi: 10.1007/s00299-024-03264-1. Plant Cell Rep. 2024. PMID: 38951279 Review.
Cited by
-
Advances in Sensing, Response and Regulation Mechanism of Salt Tolerance in Rice.Int J Mol Sci. 2021 Feb 24;22(5):2254. doi: 10.3390/ijms22052254. Int J Mol Sci. 2021. PMID: 33668247 Free PMC article. Review.
-
Autophagy receptor OsNBR1 modulates salt stress tolerance in rice.Plant Cell Rep. 2023 Dec 25;43(1):17. doi: 10.1007/s00299-023-03111-9. Plant Cell Rep. 2023. PMID: 38145426
-
Origination, Expansion, Evolutionary Trajectory, and Expression Bias of AP2/ERF Superfamily in Brassica napus.Front Plant Sci. 2016 Aug 12;7:1186. doi: 10.3389/fpls.2016.01186. eCollection 2016. Front Plant Sci. 2016. PMID: 27570529 Free PMC article.
-
Gene Overexpression Resources in Cereals for Functional Genomics and Discovery of Useful Genes.Front Plant Sci. 2016 Sep 21;7:1359. doi: 10.3389/fpls.2016.01359. eCollection 2016. Front Plant Sci. 2016. PMID: 27708649 Free PMC article. Review.
-
Catalase (CAT) Gene Family in Wheat (Triticum aestivum L.): Evolution, Expression Pattern and Function Analysis.Int J Mol Sci. 2022 Jan 4;23(1):542. doi: 10.3390/ijms23010542. Int J Mol Sci. 2022. PMID: 35008967 Free PMC article.
References
-
- Ahlfors R., Macioszek V., Rudd J., Brosché M., Schlichting R., Scheel D., Kangasjärvi J. (2004). Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 40: 512–522 - PubMed
-
- Bartels D., Sunkar R. (2005). Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 24: 23–58
-
- Bienert G.P., Schjoerring J.K., Jahn T.P. (2006). Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758: 994–1003 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

