Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses
- PMID: 23800991
- PMCID: PMC3729780
- DOI: 10.1104/pp.113.222372
Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses
Abstract
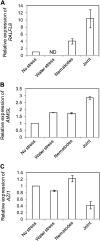
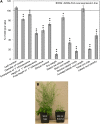
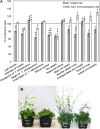
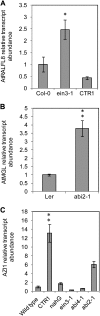
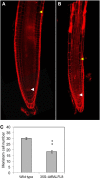
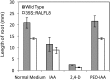
In field conditions, plants may experience numerous environmental stresses at any one time. Research suggests that the plant response to multiple stresses is different from that for individual stresses, producing nonadditive effects. In particular, the molecular signaling pathways controlling biotic and abiotic stress responses may interact and antagonize one another. The transcriptome response of Arabidopsis (Arabidopsis thaliana) to concurrent water deficit (abiotic stress) and infection with the plant-parasitic nematode Heterodera schachtii (biotic stress) was analyzed by microarray. A unique program of gene expression was activated in response to a combination of water deficit and nematode stress, with 50 specifically multiple-stress-regulated genes. Candidate genes with potential roles in controlling the response to multiple stresses were selected and functionally characterized. RAPID ALKALINIZATION FACTOR-LIKE8 (AtRALFL8) was induced in roots by joint stresses but conferred susceptibility to drought stress and nematode infection when overexpressed. Constitutively expressing plants had stunted root systems and extended root hairs. Plants may produce signal peptides such as AtRALFL8 to induce cell wall remodeling in response to multiple stresses. The methionine homeostasis gene METHIONINE GAMMA LYASE (AtMGL) was up-regulated by dual stress in leaves, conferring resistance to nematodes when overexpressed. It may regulate methionine metabolism under conditions of multiple stresses. AZELAIC ACID INDUCED1 (AZI1), involved in defense priming in systemic plant immunity, was down-regulated in leaves by joint stress and conferred drought susceptibility when overexpressed, potentially as part of abscisic acid-induced repression of pathogen response genes. The results highlight the complex nature of multiple stress responses and confirm the importance of studying plant stress factors in combination.
Figures



References
-
- Abuqamar S, Luo HL, Laluk K, Mickelbart MV, Mengiste T. (2009) Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor. Plant J 58: 347–360 - PubMed
-
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16: 3460–3479 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

