Primary breast cancer stem-like cells metastasise to bone, switch phenotype and acquire a bone tropism signature
- PMID: 23801032
- PMCID: PMC3694250
- DOI: 10.1038/bjc.2013.271
Primary breast cancer stem-like cells metastasise to bone, switch phenotype and acquire a bone tropism signature
Abstract
Background: Bone metastases represent a common and severe complication in breast cancer, and the involvement of cancer stem cells (CSCs) in the promotion of bone metastasis is currently under discussion. Here, we used a human-in-mice model to study bone metastasis formation due to primary breast CSCs-like colonisation.
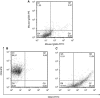
Methods: Primary CD44⁺CD24⁻ breast CSCs-like were transduced by a luciferase-lentiviral vector and injected through subcutaneous and intracardiac (IC) routes in non-obese/severe-combined immunodeficient (NOD/SCID) mice carrying subcutaneous human bone implants. The CSCs-like localisation was monitored by in vivo luciferase imaging. Bone metastatic CSCs-like were analysed through immunohistochemistry and flow cytometry, and gene expression analyses were performed by microarray techniques.
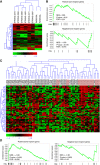
Results: Breast CSCs-like colonised the human-implanted bone, resulting in bone remodelling. Bone metastatic lesions were histologically apparent by tumour cell expression of epithelial markers and vimentin. The bone-isolated CSCs-like were CD44⁻CD24⁺ and showed tumorigenic abilities after injection in secondary mice. CD44⁻CD24⁺ CSCs-like displayed a distinct bone tropism signature that was enriched in genes that discriminate bone metastases of breast cancer from metastases at other organs.
Conclusion: Breast CSCs-like promote bone metastasis and display a CSCs-like bone tropism signature. This signature has clinical prognostic relevance, because it efficiently discriminates osteotropic breast cancers from tumour metastases at other sites.
Figures






References
-
- Abdel-Fatah TM, Powe DG, Agboola J, Adamowicz-Brice M, Blamey RW, Lopez-Garcia MA, Green AR, Reis-Filho JS, Ellis IO. The biological, clinical and prognostic implications of p53 transcriptional pathways in breast cancers. J Pathol. 2010;220 (4:419–434. - PubMed
-
- Buijs JT, van der Horst G, van den Hoogen C, Cheung H, de Rooij B, Kroon J, Petersen M, van Overveld PG, Pelger RC, van der Pluijm G. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene. 2011;31 (17:2164–2174. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

