Morphogenesis in sea urchin embryos: linking cellular events to gene regulatory network states
- PMID: 23801438
- PMCID: PMC3744329
- DOI: 10.1002/wdev.18
Morphogenesis in sea urchin embryos: linking cellular events to gene regulatory network states
Abstract
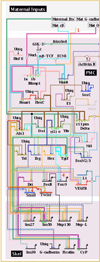
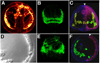
Gastrulation in the sea urchin begins with ingression of the primary mesenchyme cells (PMCs) at the vegetal pole of the embryo. After entering the blastocoel the PMCs migrate, form a syncitium, and synthesize the skeleton of the embryo. Several hours after the PMCs ingress the vegetal plate buckles to initiate invagination of the archenteron. That morphogenetic process occurs in several steps. The nonskeletogenic cells produce the initial inbending of the vegetal plate. Endoderm cells then rearrange and extend the length of the gut across the blastocoel to a target near the animal pole. Finally, cells that will form part of the midgut and hindgut are added to complete gastrulation. Later, the stomodeum invaginates from the oral ectoderm and fuses with the foregut to complete the archenteron. In advance of, and during these morphogenetic events, an increasingly complex input of transcription factors controls the specification and the cell biological events that conduct the gastrulation movements.
Copyright © 2011 Wiley Periodicals, Inc.
Figures












Similar articles
-
Pattern formation during gastrulation in the sea urchin embryo.Dev Suppl. 1992:33-41. Dev Suppl. 1992. PMID: 1299366
-
Late specification of Veg1 lineages to endodermal fate in the sea urchin embryo.Dev Biol. 1998 Mar 1;195(1):38-48. doi: 10.1006/dbio.1997.8814. Dev Biol. 1998. PMID: 9520322
-
FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development.Development. 2008 Jan;135(2):353-65. doi: 10.1242/dev.014282. Epub 2007 Dec 12. Development. 2008. PMID: 18077587
-
Ingression of primary mesenchyme cells of the sea urchin embryo: a precisely timed epithelial mesenchymal transition.Birth Defects Res C Embryo Today. 2007 Dec;81(4):241-52. doi: 10.1002/bdrc.20113. Birth Defects Res C Embryo Today. 2007. PMID: 18228256 Review.
-
Gastrulation in the sea urchin.Curr Top Dev Biol. 2020;136:195-218. doi: 10.1016/bs.ctdb.2019.08.004. Epub 2019 Oct 22. Curr Top Dev Biol. 2020. PMID: 31959288 Free PMC article. Review.
Cited by
-
Divide or Conquer: Cell Cycle Regulation of Invasive Behavior.Trends Cell Biol. 2017 Jan;27(1):12-25. doi: 10.1016/j.tcb.2016.08.003. Epub 2016 Sep 12. Trends Cell Biol. 2017. PMID: 27634432 Free PMC article. Review.
-
Architecture and evolution of the cis-regulatory system of the echinoderm kirrelL gene.Elife. 2022 Feb 25;11:e72834. doi: 10.7554/eLife.72834. Elife. 2022. PMID: 35212624 Free PMC article.
-
Developmental atlas of the indirect-developing sea urchin Paracentrotus lividus: From fertilization to juvenile stages.Front Cell Dev Biol. 2022 Oct 31;10:966408. doi: 10.3389/fcell.2022.966408. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393864 Free PMC article.
-
Lineage tracing shows that cell size asymmetries predict the dorsoventral axis in the sea star embryo.BMC Biol. 2022 Aug 15;20(1):179. doi: 10.1186/s12915-022-01359-3. BMC Biol. 2022. PMID: 35971116 Free PMC article.
-
Antagonistic BMP-cWNT signaling in the cnidarian Nematostella vectensis reveals insight into the evolution of mesoderm.Proc Natl Acad Sci U S A. 2017 Jul 11;114(28):E5608-E5615. doi: 10.1073/pnas.1701607114. Epub 2017 Jun 26. Proc Natl Acad Sci U S A. 2017. PMID: 28652368 Free PMC article.
References
-
- Angerer LM, Angerer RC. Patterning the sea urchin embryo: gene regulatory networks, signaling pathways, and cellular interactions. Curr Top Dev Biol. 2003;53:159–198. - PubMed
-
- Anstrom JA. Sea urchin primary mesenchyme cells: ingression occurs independent of microtubules. Dev Biol. 1989;131:269–275. - PubMed
-
- Anstrom JA. Microfilaments, cell shape changes, and the formation of primary mesenchyme in sea urchin embryos. J Exp Zool. 1992;264:312–322. - PubMed
-
- Anstrom JA, Chin JE, Leaf DS, Parks AL, Raff RA. Localization and expression of msp130, a primary mesenchyme lineage-specific cell surface protein in the sea urchin embryo. Development. 1987;101:255–265. - PubMed
-
- Armstrong N, Hardin J, McClay DR. Cell-cell interactions regulate skeleton formation in the sea urchin embryo. Development. 1993;119:833–840. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

