Single dose oral ibuprofen plus oxycodone for acute postoperative pain in adults
- PMID: 23801549
- PMCID: PMC6494203
- DOI: 10.1002/14651858.CD010289.pub2
Single dose oral ibuprofen plus oxycodone for acute postoperative pain in adults
Abstract
Background: Combining two different analgesics in fixed doses in a single tablet can provide better pain relief than either drug alone in acute pain. This appears to be broadly true across a range of different drug combinations, in postoperative pain and migraine headache. Fixed-dose combinations of ibuprofen and oxycodone are available, and the drugs may be separately used in combination in some acute pain situations.
Objectives: To assess the analgesic efficacy and adverse effects of a single oral dose of ibuprofen plus oxycodone for moderate to severe postoperative pain.
Search methods: We searched the Cochrane Central Register of Controlled Trials, (CENTRAL), on The Cochrane Library, (Issue 4 of 12, 2013), MEDLINE (1950 to 21st May 2013), EMBASE (1974 to 21st May 2013), the Oxford Pain Database, ClinicalTrials.gov, and reference lists of articles.
Selection criteria: Randomised, double-blind clinical trials of single dose, oral ibuprofen plus oxycodone compared with placebo or the same dose of ibuprofen alone for acute postoperative pain in adults.
Data collection and analysis: Two review authors independently considered trials for inclusion in the review, assessed quality, and extracted data. We used the area under the pain relief versus time curve to derive the proportion of participants prescribed ibuprofen plus oxycodone, ibuprofen alone, oxycodone alone, or placebo with at least 50% pain relief over six hours, using validated equations. We calculated relative risk (RR) and number needed to treat to benefit (NNT). We used information on use of rescue medication to calculate the proportion of participants requiring rescue medication and the weighted mean of the median time to use. We also collected information on adverse events.
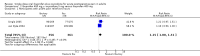
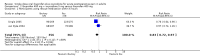
Main results: Searches identified three studies involving 1202 participants. All examined the same dose combination. Included studies provided data from 603 participants for the comparison of ibuprofen 400 mg + oxycodone 5 mg with placebo, 717 participants for the comparison of ibuprofen 400 mg + oxycodone 5 mg with ibuprofen 400 mg alone, and 471 participants for the comparison of ibuprofen 400 mg + oxycodone 5 mg with oxycodone 5 mg alone.The proportion of participants achieving at least 50% pain relief over 6 hours was 60% with ibuprofen 400 mg + oxycodone 5 mg and 17% with placebo, giving an NNT of 2.3 (2.0 to 2.8). For ibuprofen 400 mg alone the proportion was 50%, producing no significant difference between ibuprofen 400 mg + oxycodone 5 mg and ibuprofen 400 mg alone. For oxycodone 5 mg alone the proportion was 23%, giving an NNT for ibuprofen 400 mg + oxycodone 5 mg compared with oxycodone alone of 2.9 (2.3 to 4.0).Ibuprofen + oxycodone resulted in longer times to remedication than with placebo. The median time to use of rescue medication was more than 5 hours for ibuprofen 400 mg + oxycodone 5 mg, and 2.3 hours or less with placebo. Fewer participants needed rescue medication with ibuprofen + oxycodone combination than with placebo or ibuprofen alone. The proportion was 40% with ibuprofen 400 mg + oxycodone 5 mg, 83% with placebo, 53% with ibuprofen alone, and 83% with oxycodone alone, giving NNT to prevent one patient needing rescue medication of 2.4 (2.0 to 2.9), 11 (6.1 to 56), and 2.6 (2.1 to 3.4) for comparisons of ibuprofen 400 mg + oxycodone 5 mg with placebo, ibuprofen alone, and oxycodone alone, respectively.The proportion of participants experiencing one or more adverse events was 25% with ibuprofen 400 mg + oxycodone 5 mg, 25% with placebo, 26% with ibuprofen alone, and 35% with oxycodone alone; they were not significantly different. Serious adverse events were reported only after abdominal surgery 6/169 with the combination, 1/175 with ibuprofen alone, 3/52 with oxycodone alone, and 1/60 with placebo. Withdrawals for reasons other than lack of efficacy were fewer than 5% and balanced across treatment arms.
Authors' conclusions: The combination of ibuprofen 400mg + oxycodone 5mg provided analgesia for longer than oxycodone alone, but not ibuprofen alone (at the same dose). There was also a smaller chance of needing additional analgesia over about eight hours, and with no greater chance of experiencing an adverse event.
Conflict of interest statement
RAM and SD have received research support from charities, government and industry sources at various times. RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare.
Figures
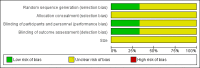


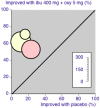







Update of
- doi: 10.1002/14651858.CD010289
References
References to studies included in this review
Litkowski 2005 {published data only}
-
- Litkowski LJ, Christensen SE, Adamson DN, Dyke T, Han SH, Newman KB. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double‐blind, placebo‐controlled, single‐dose, parallel‐group study in a dental pain model. Clinical Therapeutics 2005;27(4):418‐29. - PubMed
Singla 2005 {published data only}
-
- Singla N, Pong A, Newman K, MD‐10 Study Group. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of pain after abdominal or pelvic surgery in women: a randomized, double‐blind, placebo‐ and active‐controlled parallel‐group study. Clinical Theraputics 2005;27(1):45‐57. - PubMed
van Dyke 2004 {published data only}
-
- Dyke T, Litkowski LJ, Kiersch TA, Zarringhalam NM, Zheng H, Newman K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double‐blind, placebo‐ and active‐controlled parallel‐group study. Clinical Therapeutics 2004;26(12):2003‐14. - PubMed
Additional references
Barden 2004
Chaparro 2012
Collins 1997
Collins 2001
Cook 1995
Cooper 1991
-
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The Design of Analgesic Clinical Trials. Advances in Pain Research and Therapy. Vol. 18, New York: Raven Press, 1991:117‐24.
Derry 2009
Derry 2010
Edwards 2002
FitzGerald 2001
Forrest 2002
Gaskell 2009
Hernandez‐Diaz 2001
Higgins 2011
-
- Altman DG, Antes G, Gøtzsche P, Higgins JPT, Jüni P, Lewis S, et al. Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Jadad 1996a
Jadad 1996b
Kalso 2007
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
McQuay 2005
Moher 1999
Moore 1996
Moore 1997a
Moore 1997b
Moore 1998
Moore 2003
-
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7]
Moore 2005
Moore 2006
-
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2]
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐23. [ISBN: 978‐0‐931092‐69‐5]
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2012
Morris 1995
Nüesch 2010
Ordóñez Gallego 2007
Poyhia 1993
-
- Poyhia R, Vainio A, Kalso E. A review of oxycodone's clinical pharmacokinetics and pharmacodynamics. Journal of Pain & Symptom Management 1993;8(2):63‐7. - PubMed
Rapoport 1999
-
- Rapoport RJ. The safety of NSAIDs and related drugs for the management of acute pain: maximizing benefits and minimizing risks. Cancer Control 1999;6(2 Suppl 1):18‐21. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Toms 2008
Toms 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

