Chemical synthesis of hydrocarbon-stapled peptides for protein interaction research and therapeutic targeting
- PMID: 23801563
- PMCID: PMC4879976
- DOI: 10.1002/9780470559277.ch110042
Chemical synthesis of hydrocarbon-stapled peptides for protein interaction research and therapeutic targeting
Abstract
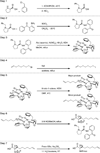
The peptide α-helix represents one of nature's most featured protein shapes and is employed in a diversity of protein architectures, from the cytoskeletal infrastructure to the most intimate contact points between crucial signaling proteins. By installing an all-hydrocarbon crosslink into native sequences, the shape and biological activity of natural peptide α-helices can be recapitulated, yielding a chemical toolbox that can be used both to interrogate the protein interactome and to modulate interaction networks for potential therapeutic benefit. Here, current methodology for synthesizing stabilized α-helices (SAH) corresponding to key protein interaction domains is described. A stepwise approach is taken for the production of crosslinking non-natural amino acids, incorporation of the residues into peptide templates, and application of ruthenium-catalyzed ring-closing metathesis to generate hydrocarbon-stapled peptides. Through facile derivatization and functionalization steps, SAHs can be tailored for a broad range of applications in biochemical, structural, proteomic, cellular, and in vivo studies. Curr. Protoc. Chem. Biol. 3:99-117 © 2011 by John Wiley & Sons, Inc.
Keywords: hydrocarbon stapling; olefin metathesis; peptide; photoreactive; protein interaction; targeting; α‐helix.
Figures



References
-
- Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. - PubMed
-
- Belokon Y, Tararov V, Maleev V, Savel’eva T, Ryzhov M. Improved procedures for the synthesis of (S)-2-[N-(N′-benzylprolyl)amino]benzophenone (BPB) and Ni(II) complexes of Schiff's bases derived from BPB and amino acids. Tetrahedron: Asymmetry. 1998;9:4249–4252.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

