Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial
- PMID: 23801579
- PMCID: PMC3806608
- DOI: 10.2337/db13-0236
Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial
Abstract
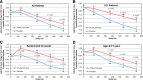
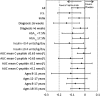
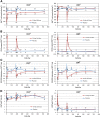
Protégé was a phase 3, randomized, double-blind, parallel, placebo-controlled 2-year study of three intravenous teplizumab dosing regimens, administered daily for 14 days at baseline and again after 26 weeks, in new-onset type 1 diabetes. We sought to determine efficacy and safety of teplizumab immunotherapy at 2 years and to identify characteristics associated with therapeutic response. Of 516 randomized patients, 513 were treated, and 462 completed 2 years of follow-up. Teplizumab (14-day full-dose) reduced the loss of C-peptide mean area under the curve (AUC), a prespecified secondary end point, at 2 years versus placebo. In analyses of prespecified and post hoc subsets at entry, U.S. residents, patients with C-peptide mean AUC >0.2 nmol/L, those randomized ≤6 weeks after diagnosis, HbA1c <7.5% (58 mmol/mol), insulin use <0.4 units/kg/day, and 8-17 years of age each had greater teplizumab-associated C-peptide preservation than their counterparts. Exogenous insulin needs tended to be reduced versus placebo. Antidrug antibodies developed in some patients, without apparent change in drug efficacy. No new safety or tolerability issues were observed during year 2. In summary, anti-CD3 therapy reduced C-peptide loss 2 years after diagnosis using a tolerable dose.
Figures
Comment in
-
Depleting T cells in newly diagnosed autoimmune (type 1) diabetes--are we getting anywhere?Diabetes. 2013 Nov;62(11):3669-70. doi: 10.2337/db13-1207. Diabetes. 2013. PMID: 24158996 Free PMC article. No abstract available.
References
-
- Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes 2004;53:426–433 - PubMed
-
- Binder C, Faber OK. Residual beta-cell function and its metabolic consequences. Diabetes 1978;27(Suppl. 1):226–229 - PubMed
-
- Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 - PubMed
-
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 2006;55:3238–3245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

