Validity of diagnostic codes to identify cases of severe acute liver injury in the US Food and Drug Administration's Mini-Sentinel Distributed Database
- PMID: 23801638
- PMCID: PMC4409951
- DOI: 10.1002/pds.3470
Validity of diagnostic codes to identify cases of severe acute liver injury in the US Food and Drug Administration's Mini-Sentinel Distributed Database
Abstract
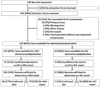
Purpose: The validity of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to identify diagnoses of severe acute liver injury (SALI) is not well known. We examined the positive predictive values (PPVs) of hospital ICD-9-CM diagnoses in identifying SALI among health plan members in the Mini-Sentinel Distributed Database (MSDD) for patients without liver/biliary disease and for those with chronic liver disease (CLD).
Methods: We selected random samples of members (149 without liver/biliary disease; 75 with CLD) with a principal hospital diagnosis suggestive of SALI (ICD-9-CM 570, 572.2, 572.4, 572.8, 573.3, 573.8, or V42.7) in the MSDD (2009-2010). Medical records were reviewed by hepatologists to confirm SALI events. PPVs of codes and code combinations for confirmed SALI were determined by CLD status.
Results: Among 105 members with available records and no liver/biliary disease, SALI was confirmed in 26 (PPV, 24.7%; 95%CI, 16.9-34.1%). Combined hospital diagnoses of acute hepatic necrosis (570) and liver disease sequelae (572.8) had high PPV (100%; 95%CI, 59.0-100%) and identified 7/26 (26.9%) events. Among 46 CLD members with available records, SALI was confirmed in 19 (PPV, 41.3%; 95%CI, 27.0-56.8%). Acute hepatic necrosis (570) or hepatorenal syndrome (572.4) plus any other SALI code had a PPV of 83.3% (95%CI, 51.6-97.9%) and identified 10/19 (52.6%) events.
Conclusions: Most individual hospital ICD-9-CM diagnoses had low PPV for confirmed SALI events. Select code combinations had high PPV but did not capture all events.
Keywords: ICD-9 codes; hepatotoxicity; liver injury; pharmacoepidemiology; validity.
Copyright © 2013 John Wiley & Sons, Ltd.
Conflict of interest statement
All authors report no potential conflicts of interest related to this manuscript.
Figures

References
-
- [Accessed February 7, 2009];PhRMA/FDA/AASLD Drug-Induced Hepatotoxicity White Paper Postmarketing Considerations. 2000 Nov; http://www.fda.gov/cder/livertox/postmarket.pdf.
-
- Kaplowitz N. Drug-induced liver disorders: implications for drug development and regulation. Drug Saf. 2001;24(7):483–490. - PubMed
-
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003 Jul 31;349(5):474–485. - PubMed
-
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006 Feb 16;354(7):731–739. - PubMed
-
- Temple RJ, Himmel MH. Safety of newly approved drugs: implications for prescribing. JAMA. 2002 May 1;287(17):2273–2275. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

