AMPK: a target for drugs and natural products with effects on both diabetes and cancer
- PMID: 23801715
- PMCID: PMC3712072
- DOI: 10.2337/db13-0368
AMPK: a target for drugs and natural products with effects on both diabetes and cancer
Abstract
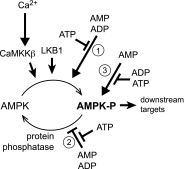
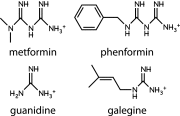
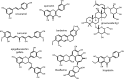
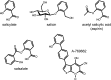
The AMP-activated protein kinase (AMPK) is a highly conserved sensor of cellular energy that appears to have arisen at an early stage during eukaryotic evolution. In 2001 it was shown to be activated by metformin, currently the major drug for treatment for type 2 diabetes. Although the known metabolic effects of AMPK activation are consistent with the idea that it mediates some of the therapeutic benefits of metformin, as discussed below it now appears unlikely that AMPK is the sole target of the drug. AMPK is also activated by several natural plant products derived from traditional medicines, and the mechanisms by which they activate AMPK are discussed. One of these is salicylate, probably the oldest medicinal agent known to humankind. The salicylate prodrug salsalate has been shown to improve metabolic parameters in subjects with insulin resistance and prediabetes, and whether this might be mediated in part by AMPK is discussed. Interestingly, there is evidence that both metformin and aspirin provide some protection against development of cancer in humans, and whether AMPK might be involved in these effects is also discussed.
Figures
References
-
- Hardie DG, Carling D, Sim ATR. The AMP-activated protein kinase—a multisubstrate regulator of lipid metabolism. Trends Biochem Sci 1989;14:20–23
-
- Hawley SA, Davison M, Woods A, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 1996;271:27879–27887 - PubMed
-
- Davies SP, Sim ATR, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem 1990;187:183–190 - PubMed
-
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007;8:774–785 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

