Potential function of granulysin, other related effector molecules and lymphocyte subsets in patients with TB and HIV/TB coinfection
- PMID: 23801887
- PMCID: PMC3691799
- DOI: 10.7150/ijms.6437
Potential function of granulysin, other related effector molecules and lymphocyte subsets in patients with TB and HIV/TB coinfection
Abstract
Background: Host effector mechanism against Mycobacterium tuberculosis (Mtb) infection is dependent on innate immune response by macrophages and neutrophils and the alterations in balanced adaptive immunity. Coordinated release of cytolytic effector molecules from NK cells and effector T cells and the subsequent granule-associated killing of infected cells have been documented; however, their role in clinical tuberculosis (TB) is still controversy.
Objective: To investigate whether circulating granulysin and other effector molecules are associated with the number of NK cells, iNKT cells, Vγ9(+)Vδ2(+) T cells, CD4(+) T cells and CD8(+) T cells, and such association influences the clinical outcome of the disease in patients with pulmonary TB and HIV/TB coinfection.
Methods: Circulating granulysin, perforin, granzyme-B and IFN-γ levels were determined by ELISA. The isoforms of granulysin were analyzed by Western blot analysis. The effector cells were analyzed by flow cytometry.
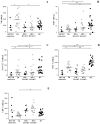
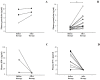
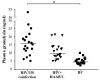
Results: Circulating granulysin and perforin levels in TB patients were lower than healthy controls, whereas the granulysin levels in HIV/TB coinfection were much higher than in any other groups, TB and HIV with or without receiving HAART, which corresponded to the number of CD8(+) T cells which kept high, but not with NK cells and other possible cellular sources of granulysin. In addition, the 17kDa, 15kDa and 9kDa isoforms of granulysin were recognized in plasma of HIV/TB coinfection. Increased granulysin and decreased IFN-γ levels in HIV/TB coinfection and TB after completion of anti-TB therapy were observed.
Conclusion: The results suggested that the alteration of circulating granulysin has potential function in host immune response against TB and HIV/TB coinfection. This is the first demonstration so far of granulysin in HIV/TB coinfection.
Keywords: Granulysin; HIV; HIV/TB Coinfection; Lymphocytes Subsets.; TB.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Coordinated In Vitro Release of Granulysin, Perforin and IFN-γ in TB and HIV/TB Co-Infection Associated with Clinical Outcomes before and after Anti-TB Treatment.Pathogens. 2020 Aug 14;9(8):655. doi: 10.3390/pathogens9080655. Pathogens. 2020. PMID: 32823923 Free PMC article.
-
Plasma granulysin levels and cellular interferon-gamma production correlate with curative host responses in tuberculosis, while plasma interferon-gamma levels correlate with tuberculosis disease activity in adults.Tuberculosis (Edinb). 2007 Jul;87(4):312-21. doi: 10.1016/j.tube.2007.01.002. Epub 2007 Mar 26. Tuberculosis (Edinb). 2007. PMID: 17382591
-
Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis.Infect Immun. 2007 Nov;75(11):5210-22. doi: 10.1128/IAI.00624-07. Epub 2007 Jul 30. Infect Immun. 2007. PMID: 17664265 Free PMC article.
-
Thinking Outside the Box: Innate- and B Cell-Memory Responses as Novel Protective Mechanisms Against Tuberculosis.Front Immunol. 2020 Feb 14;11:226. doi: 10.3389/fimmu.2020.00226. eCollection 2020. Front Immunol. 2020. PMID: 32117325 Free PMC article. Review.
-
Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis.Curr Opin HIV AIDS. 2012 May;7(3):268-75. doi: 10.1097/COH.0b013e3283524e32. Curr Opin HIV AIDS. 2012. PMID: 22495739 Review.
Cited by
-
Host Antimicrobial Peptides: The Promise of New Treatment Strategies against Tuberculosis.Front Immunol. 2017 Nov 7;8:1499. doi: 10.3389/fimmu.2017.01499. eCollection 2017. Front Immunol. 2017. PMID: 29163551 Free PMC article. Review.
-
The assessment of host and bacterial proteins in sputum from active pulmonary tuberculosis.J Microbiol. 2016 Nov;54(11):761-767. doi: 10.1007/s12275-016-6201-x. Epub 2016 Oct 29. J Microbiol. 2016. PMID: 27796930
-
Mycobacterium tuberculosis Co-operonic PE32/PPE65 Proteins Alter Host Immune Responses by Hampering Th1 Response.Front Microbiol. 2016 May 17;7:719. doi: 10.3389/fmicb.2016.00719. eCollection 2016. Front Microbiol. 2016. PMID: 27242739 Free PMC article.
-
Granulysin: killer lymphocyte safeguard against microbes.Curr Opin Immunol. 2019 Oct;60:19-29. doi: 10.1016/j.coi.2019.04.013. Epub 2019 May 18. Curr Opin Immunol. 2019. PMID: 31112765 Free PMC article. Review.
-
Granulysin as a novel factor for the prognosis of the clinical course of chickenpox.Epidemiol Infect. 2018 May;146(7):854-857. doi: 10.1017/S0950268818000717. Epub 2018 Apr 10. Epidemiol Infect. 2018. PMID: 29633679 Free PMC article.
References
-
- Global Tuberculosis report 2012. Geneva, Switzerland: WHO; http://www.who.int/tb/publications/global_report/en/
-
- Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. - PubMed
-
- Goletti D, Weissman D, Jackson RW. et al. Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol. 1996;157:1271–8. - PubMed
-
- Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: How CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

