Competence and natural transformation in vibrios
- PMID: 23803158
- PMCID: PMC3820095
- DOI: 10.1111/mmi.12307
Competence and natural transformation in vibrios
Abstract
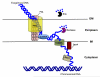
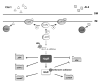
Natural transformation is a major mechanism of horizontal gene transfer in bacteria. By incorporating exogenous DNA elements into chromosomes, bacteria are able to acquire new traits that can enhance their fitness in different environments. Within the past decade, numerous studies have revealed that natural transformation is prevalent among members of the Vibrionaceae, including the pathogen Vibrio cholerae. Four environmental factors: (i) nutrient limitation, (ii) availability of extracellular nucleosides, (iii) high cell density and (iv) the presence of chitin, promote genetic competence and natural transformation in Vibrio cholerae by co-ordinating expression of the regulators CRP, CytR, HapR and TfoX respectively. Studies of other Vibrionaceae members highlight the general importance of natural transformation within this bacterial family.
© 2013 John Wiley & Sons Ltd.
Figures



References
-
- Aas FE, Wolfgang M, Frye S, Dunham S, Løvold C, Koomey M. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol Microbiol. 2002;46:749–760. - PubMed
-
- Anorsti C. Microbial extracellular enzymes and the marine carbon cycle. Ann Rev Mar Sci. 2011;3:401–425. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

