Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error
- PMID: 23803325
- PMCID: PMC3763153
- DOI: 10.1152/jn.00609.2012
Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error
Abstract
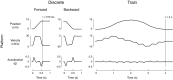
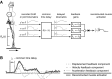
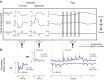
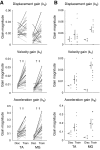
In both the upper and lower limbs, evidence suggests that short-latency electromyographic (EMG) responses to mechanical perturbations are modulated based on muscle stretch or joint motion, whereas long-latency responses are modulated based on attainment of task-level goals, e.g., desired direction of limb movement. We hypothesized that long-latency responses are modulated continuously by task-level error feedback. Previously, we identified an error-based sensorimotor feedback transformation that describes the time course of EMG responses to ramp-and-hold perturbations during standing balance (Safavynia and Ting 2013; Welch and Ting 2008, 2009). Here, our goals were 1) to test the robustness of the sensorimotor transformation over a richer set of perturbation conditions and postural states; and 2) to explicitly test whether the sensorimotor transformation is based on task-level vs. joint-level error. We developed novel perturbation trains of acceleration pulses such that perturbations were applied when the body deviated from the desired, upright state while recovering from preceding perturbations. The entire time course of EMG responses (∼4 s) in an antagonistic muscle pair was reconstructed using a weighted sum of center of mass (CoM) kinematics preceding EMGs at long-latency delays (∼100 ms). Furthermore, CoM and joint kinematic trajectories became decorrelated during perturbation trains, allowing us to explicitly compare task-level vs. joint feedback in the same experimental condition. Reconstruction of EMGs was poorer using joint kinematics compared with CoM kinematics and required unphysiologically short (∼10 ms) delays. Thus continuous, long-latency feedback of task-level variables may be a common mechanism regulating long-latency responses in the upper and lower limbs.
Keywords: balance; electromyography; postural control; reflex; sensorimotor response.
Figures


Similar articles
-
Center of mass states render multijoint torques throughout standing balance recovery.J Neurophysiol. 2025 Jan 1;133(1):206-221. doi: 10.1152/jn.00367.2024. Epub 2024 Dec 10. J Neurophysiol. 2025. PMID: 39658948
-
Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies.J Neurophysiol. 2013 Jan;109(1):31-45. doi: 10.1152/jn.00684.2012. Epub 2012 Oct 24. J Neurophysiol. 2013. PMID: 23100133 Free PMC article.
-
Rapid and flexible whole body postural responses are evoked from perturbations to the upper limb during goal-directed reaching.J Neurophysiol. 2017 Mar 1;117(3):1070-1083. doi: 10.1152/jn.01004.2015. Epub 2016 Dec 21. J Neurophysiol. 2017. PMID: 28003415 Free PMC article.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
Review of balance recovery in response to external perturbations during daily activities.Hum Mov Sci. 2020 Feb;69:102546. doi: 10.1016/j.humov.2019.102546. Epub 2019 Dec 31. Hum Mov Sci. 2020. PMID: 31989948 Review.
Cited by
-
Center of mass states render multijoint torques throughout standing balance recovery.J Neurophysiol. 2025 Jan 1;133(1):206-221. doi: 10.1152/jn.00367.2024. Epub 2024 Dec 10. J Neurophysiol. 2025. PMID: 39658948
-
Evidence for constancy in the modularity of trunk muscle activity preceding reaching: implications for the role of preparatory postural activity.J Neurophysiol. 2021 Nov 1;126(5):1465-1477. doi: 10.1152/jn.00093.2021. Epub 2021 Sep 29. J Neurophysiol. 2021. PMID: 34587462 Free PMC article.
-
Center of mass kinematic reconstruction during steady-state walking using optimized template models.PLoS One. 2024 Nov 5;19(11):e0313156. doi: 10.1371/journal.pone.0313156. eCollection 2024. PLoS One. 2024. PMID: 39499675 Free PMC article.
-
Mechanisms of motor adaptation in reactive balance control.PLoS One. 2014 May 8;9(5):e96440. doi: 10.1371/journal.pone.0096440. eCollection 2014. PLoS One. 2014. PMID: 24810991 Free PMC article.
-
Lower extremity joint-level responses to pelvis perturbation during human walking.Sci Rep. 2018 Oct 2;8(1):14621. doi: 10.1038/s41598-018-32839-8. Sci Rep. 2018. PMID: 30279499 Free PMC article.
References
-
- Abrahamova D, Hlavacka F. Age-related changes of human balance during quiet stance. Physiol Res 57: 957–964, 2008 - PubMed
-
- Allum JH, Honegger F. Interactions between vestibular and proprioceptive inputs triggering and modulating human balance-correcting responses differ across muscles. Exp Brain Res 121: 478–494, 1998 - PubMed
-
- Basmajian JV, Blumenstein R, Dismatsek M. Electrode Placement in EMG Biofeedback. Baltimore, MD: Williams and Wilkins, 1980
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

