Membrane damage by an α-helical pore-forming protein, Equinatoxin II, proceeds through a succession of ordered steps
- PMID: 23803608
- PMCID: PMC3745318
- DOI: 10.1074/jbc.M113.481572
Membrane damage by an α-helical pore-forming protein, Equinatoxin II, proceeds through a succession of ordered steps
Abstract
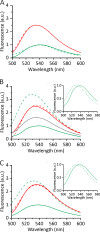
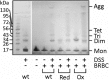
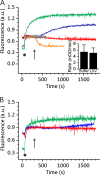
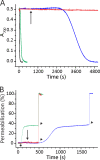
Actinoporin equinatoxin II (EqtII) is an archetypal example of α-helical pore-forming toxins that porate cellular membranes by the use of α-helices. Previous studies proposed several steps in the pore formation: binding of monomeric protein onto the membrane, followed by oligomerization and insertion of the N-terminal α-helix into the lipid bilayer. We studied these separate steps with an EqtII triple cysteine mutant. The mutant was engineered to monitor the insertion of the N terminus into the lipid bilayer by labeling Cys-18 with a fluorescence probe and at the same time to control the flexibility of the N-terminal region by the disulfide bond formed between cysteines introduced at positions 8 and 69. The insertion of the N terminus into the membrane proceeded shortly after the toxin binding and was followed by oligomerization. The oxidized, non-lytic, form of the mutant was still able to bind to membranes and oligomerize at the same level as the wild-type or the reduced form. However, the kinetics of the N-terminal helix insertion, the release of calcein from erythrocyte ghosts, and hemolysis of erythrocytes was much slower when membrane-bound oxidized mutant was reduced by the addition of the reductant. Results show that the N-terminal region needs to be inserted in the lipid membrane before the oligomerization into the final pore and imply that there is no need for a stable prepore formation. This is different from β-pore-forming toxins that often form β-barrel pores via a stable prepore complex.
Keywords: Actinoporin; Equinatoxin; Erythrocyte; Fluorescence; Kinetics; Membrane; Pore Forming; Toxins.
Figures


References
-
- Parker M. W., Feil S. C. (2005) Pore-forming protein toxins. From structure to function. Prog. Biophys. Mol. Biol. 88, 91–142 - PubMed
-
- Anderluh G., Lakey J. H. (2008) Disparate proteins use similar architectures to damage membranes. Trends Biochem. Sci. 33, 482–490 - PubMed
-
- Dalla Serra M., Tejuca Martinez M. (2011) Pore-forming toxins. eLS, DOI: 10.1002/9780470015902.a0002655.pub2 - DOI
-
- Heuck A. P., Tweten R. K., Johnson A. E. (2001) β-barrel pore-forming toxins. Intriguing dimorphic proteins. Biochemistry 40, 9065–9073 - PubMed
-
- Mueller M., Grauschopf U., Maier T., Glockshuber R., Ban N. (2009) The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature 459, 726–730 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

