Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors
- PMID: 23803693
- PMCID: PMC3845888
- DOI: 10.1093/carcin/bgt231
Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors
Abstract
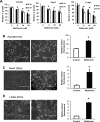
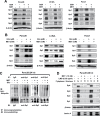
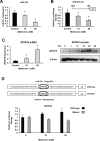
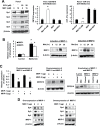
Metformin is a widely used antidiabetic drug, and epidemiology studies for pancreatic and other cancers indicate that metformin exhibits both chemopreventive and chemotherapeutic activities. Several metformin-induced responses and genes are similar to those observed after knockdown of specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 by RNA interference, and we hypothesized that the mechanism of action of metformin in pancreatic cancer cells was due, in part, to downregulation of Sp transcription factors. Treatment of Panc1, L3.6pL and Panc28 pancreatic cancer cells with metformin downregulated Sp1, Sp3 and Sp4 proteins and several pro-oncogenic Sp-regulated genes including bcl-2, survivin, cyclin D1, vascular endothelial growth factor and its receptor, and fatty acid synthase. Metformin induced proteasome-dependent degradation of Sps in L3.6pL and Panc28 cells, whereas in Panc1 cells metformin decreased microRNA-27a and induced the Sp repressor, ZBTB10, and disruption of miR-27a:ZBTB10 by metformin was phosphatase dependent. Metformin also inhibited pancreatic tumor growth and downregulated Sp1, Sp3 and Sp4 in tumors in an orthotopic model where L3.6pL cells were injected directly into the pancreas. The results demonstrate for the first time that the anticancer activities of metformin are also due, in part, to downregulation of Sp transcription factors and Sp-regulated genes.
Figures


References
-
- Bolen S., et al. (2007). Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann. Intern. Med., 147, 386–399 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

