Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP)
- PMID: 23803961
- PMCID: PMC4604951
Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP)
Abstract
On February 20, 2013, the Advisory Committee on Immunization Practices (ACIP) recommended routine use of 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13, Wyeth Pharmaceuticals, Inc., a subsidiary of Pfizer, Inc.) for children aged 6-18 years with immunocompromising conditions, functional or anatomic asplenia, cerebrospinal fluid (CSF) leaks, or cochlear implants who have not previously received PCV13. PCV13 should be administered to these children regardless of whether they received the 7-valent pneumococcal conjugate vaccine (PCV7) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Recommendations for PPSV23 use for children in this age group remain unchanged. The evidence for the benefits and risks associated with PCV13 vaccination of children with immunocompromising conditions was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework. This recommendation reflects a policy change from permissive and off-label recommendation of PCV13 in the pediatric immunocompromised population to a category A recommendation. This report summarizes the evidence considered by ACIP to make this recommendation and reviews the recommendations for use of PCV13 and PPSV23 for children aged 6-18 years.
Figures
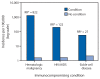
References
-
- CDC. GRADE evidence tables—recommendations in MMWR. Atlanta, GA: US Department of Health and Human Services, CDC; 2013. Available at http://www.cdc.gov/vaccines/acip/recs/grade/table-refs.html.
-
- Ahmed F, Temte JL, Campos-Outcalt D, Schunemann HJ ACIP Evidence Based Recommendations Work Group. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC) Vaccine. 2011;29:9171–6. - PubMed
-
- Huang SS, Johnson KM, Ray GT, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–412. - PubMed
-
- CDC. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59(RR-11) - PubMed
-
- Cohen AL, Harrison LH, Farley MM, et al. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS. 2010;24:2253–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

