Emergence of orientation selectivity in the Mammalian visual pathway
- PMID: 23804085
- PMCID: PMC3693051
- DOI: 10.1523/JNEUROSCI.0404-13.2013
Emergence of orientation selectivity in the Mammalian visual pathway
Abstract
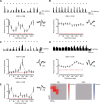
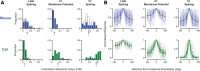
Orientation selectivity is a property of mammalian primary visual cortex (V1) neurons, yet its emergence along the visual pathway varies across species. In carnivores and primates, elongated receptive fields first appear in V1, whereas in lagomorphs such receptive fields emerge earlier, in the retina. Here we examine the mouse visual pathway and reveal the existence of orientation selectivity in lateral geniculate nucleus (LGN) relay cells. Cortical inactivation does not reduce this orientation selectivity, indicating that cortical feedback is not its source. Orientation selectivity is similar for LGN relay cells spiking and subthreshold input to V1 neurons, suggesting that cortical orientation selectivity is inherited from the LGN in mouse. In contrast, orientation selectivity of cat LGN relay cells is small relative to subthreshold inputs onto V1 simple cells. Together, these differences show that although orientation selectivity exists in visual neurons of both rodents and carnivores, its emergence along the visual pathway, and thus its underlying neuronal circuitry, is fundamentally different.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
